© Springer Nature Singapore Pte Ltd. 2017
Jia Wei and Baorui Liu (eds.)Personalized Management of Gastric Cancer10.1007/978-981-10-3978-2_11. Molecular Pathology of Heredity Gastric Cancer
(1)
Department of Pathology of Drum Tower Hospital, Medical School of Nanjing University, Nanjing, 210008, China
1.1 Introduction
Gastric cancer (GC) affects nearly one million individuals every year, and most of them are from China, Japan, and Korea. It is the fifth most common malignant tumor worldwide and the third leading cause of malignant tumor mortality with more than 723,000 deaths [1]. About 70–85% of individuals with GC die within 5 years of diagnosis, and the high mortality associated with GC is mainly a result of limited therapeutic methods and lacking of early diagnosis. Aggregation within families occurs in almost 10% of patients (5–30%), although most GCs are sporadic. Now we know that hereditary germline mutations lead to half of these familial cases [2, 3]. In regions where the incidence of GC is low, heritable pathogenic mutations, which leads to most familial cases, increase risk from birth. Truly hereditary cases, as some studies pointed out, account for 1–3% of the global burden of GC [4], and most of those are hereditary diffuse gastric cancer (HDGC). It is reported that, in about 40% of families affected by HDGC, the E-cadherin/CDH1 germline mutations can been found. It is very important to identify the inherited factors among patients with family histories of GC, in order to diagnose early and manage effectively. We usually use symptoms, such as different family individuals are diagnosed with cancer, the histological types are diffused adenocarcinoma, and the patients are young and with multiple cancer syndromes, to identify HDGC. Some cases of other hereditary tumor syndromes may also present GC, and thus the risk of GC should be taken into account in these patients. The hereditary cancer syndromes include the gastric adenocarcinoma and proximal polyposis of the stomach (GAPPS), familial intestinal gastric cancer (FIGC), Lynch syndrome (LS) caused by germline mutations in DNA mismatch repair genes and microsatellite instability, familial adenomatous polyposis (FAP) associated with germline APC mutations, MUTYH-associated polyposis (MAP) associated with MUTYH mutation, Peutz-Jeghers syndrome (PJS) caused by germline STK11 mutations , juvenile polyposis syndrome (JPS) associated with germline mutations in the BMPR1A and SMAD4 genes, hereditary breast-ovarian cancer syndromes (HBCS) related to germline mutations of BRCA1 and BRCA2, Li-Fraumeni syndrome (LFS) due to germline p53 mutations, and so on [5].
Screening for familial gastric cancer (FGC) is an especially important procedure. Because it has a higher risk of GC incidence, to the individuals who have inherited the mutant gene, prophylactic gastrectomy is worthy of consideration [6]. It is an enormous fiscal expenditure of society to manage and control FGC each year. Thus, screening for prevention for it is a crucial step to decrease cancer incidence and mortality [7]. It is necessary to interview with pedigree precisely to find the familial syndromes, individuals at risk, and genotypes [8]. At present, it is in urgent need of guidelines for genetic detecting, counseling, and management of patients with HDGC. If we pay more attention on these syndromes, we may increase opportunities to detect and prevent GCs in these high-risk cases.
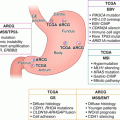
Hereditary gastric cancer syndromes are an infrequent but characteristic etiology of GCs. So far, we haven’t clarified the genetic mutations attacking most affected families. Up to date, there are at least three main hereditary GC syndromes that have been reported: HDGC, GAPPS, and FIGC [5, 9]. In this chapter, we mainly discuss the available knowledge on HDGC, GAPPS, FIGC, and several other hereditary cancer syndromes associated with GC, with the aim of clarifying the molecular pathology and genesis of these heredity GCs.
1.2 Hereditary Diffuse Gastric Cancer
HDGC is an autosomal dominant disorder predisposition syndrome with obvious penetrance (about 80%) and characterized by an enhancive risk of early-onset, multigenerational, and signet ring cell GC (Lauren diffuse type) and lobular breast carcinoma.
A diagnosis of families with the HDGC syndrome can be established if one of the following clinical features are present. Firstly, at least two patients of documented diffuse GC in first- or second-degree family members, with one or more being diagnosed younger than 50 years old. Secondly, at least three documented patients of diffuse GC in first- or second-degree family members, ignoring of age of diagnosis. The checklist above was defined by the International Gastric Cancer Linkage Consortium (IGCLC) in 1999.
There was a renewed version for genetic testing in 2010 [10]. The families, which fulfill the following criteria for HDGC, would be recommended to consider genetic counseling and genetic testing for CDH1 gene mutations. Firstly, at least two patients of documented diffuse GC in first-degree family members, with one or more documented case of diffuse GC being diagnosed younger than 50 years old. Secondly, at least three documented patients of diffuse GC in first- or second-degree family members, ignoring of age of diagnosis. Thirdly, the diffuse GC case, with no family history, was diagnosed before the age of 40 years. Fourthly, families with patients of both lobular breast cancer and diffuse GC, with at least one diagnosed younger than the age of 50 years.
The age at onset of clinically significant diffuse GC may be extremely variable with the average onset in the fourth decade of life (14–85 years old), and the distribution of lesions also varies, involving all the topographic regions within the stomach. HDGC’s genetic susceptibility and the molecular basis were first identified and then reinforced in Maori families and other populations, respectively, in 1998 [11, 12].
Heterozygous germline alterations in the E-cadherin gene (CDH1) result in HDGC [11]. There are five types of germline CDH1 mutations, including large rearrangements (4–8.7%), nonsense (17.3%) and missense (17.3%) mutations, splice site (23.1%), as well as small frameshifts (37.5%). They affect the protein’s functional domains and the entire coding sequence [13–15]. 141 probands harboring more than one hundred different pathogenic germline CDH1 alterations have been described across multiple ethnicities so far [16, 17]. The production of this gene, which locates at 16q22.1, is E-cadherin. E-cadherin is a calcium-dependent transmembrane cellular adhesion protein. There are three parts of E-cadherin, including an intracellular domain which binds β-catenin and p120-catenin, the transmembrane domain, and the extracellular domain with five cadherin repeats (EC1–EC5). There is a highly phosphorylated region in the intracellular domain. Binding β-catenin with the intracellular domain is necessary for E-cadherin to regulate intracellular signaling. E-cadherin promotes tumor growth by interacting with cytoskeleton actin filaments through the Wnt signaling pathway. Downregulation of E-cadherin is observed in a lot of epithelial carcinomas of human and promotes invasion through loss of epithelial cell-cell adhesion. Being deprived of E-cadherin expression or function has been involved in cancer progression and metastasis. Around 40% of the families with HDGC have CDH1 germline mutations with high susceptibility to early development of diffuse-type GC [11]. The frequency of CDH1 germline mutations was found to be much lower in families from the high incidence of GC regions (i.e., the Eastern) (13.0%) than in countries with low incidence of GC (i.e., New Zealand, Northern Europe, and North America) (26.8%) [18]. About 45.6% cases of HDGC probands carried CDH1 germline alterations [14]. However, only 5.7% [14] to 13.8% [18] of FDGC probands displayed mutations of CDH1 but not large deletions. Very rarely, germline promoter methylation of CDH1 or inherited a-E-catenin mutations has been reported recently [19].
The cases, who just have a single wild-type CDH1 allele, are called heterozygous carriers. Usually these patients are commonly asymptomatic because the functional CDH1 gene produces sufficient amount of E-cadherin protein to maintain all normal functions in the stomach [20]. When the remaining wild-type allele of the E-cadherin gene is inactivated by a second-hit molecular mechanism until the second decade of life, HDGC develops. There are mainly three types of second-hit mechanism of inactivation in both inherited and sporadic diffuse cancers, including the epigenetic modification (promoter hypermethylation of CDH1), deletion (LOH or intragenic deletions), or a second mutation [21, 22]. The former is the most common one. Primary tumor and lymph node metastases may show different second-hit mechanisms or different tumorous lesions and even the same neoplastic lesion from the same patient. For example, LOH is often found in lymph node metastases (58.3%), whereas promoter hypermethylation of CDH1 mainly occurs in primary lesions [15]. Genetic testing of CDH1 is recommended in a patient fulfilling the HDGC diagnose checklist above. The other families that met the IGCLC criteria remained genetically unexplained. Recently, candidate mutations were identified in 11% (16/144) probands in those CDH1 mutation-negative index cases, including mutations within genes of moderate and high penetrance: STK11, SDHB, PRSS1, ATM, CTNNA1, MSR1, BRCA2, and PALB2 [23].
There are two major histological variants of GC: diffuse-type GC (35%) and intestinal-type GC (50%). CDH1 mutations have been found only in diffuse-type GCs. Microscopically, single or multiple foci of invasive signet ring cells just develop below the surface mucosal epithelium, retaining the construction. On the other hand, cancer cells may show a pattern of signet ring cell carcinoma in situ and pagetoid distribution below the conserved epithelium of foveolae and pits but still be restricted to the basement membrane. Usually advanced HDGC shows as a poorly cohesive malignant tumor, while the entire stomach wall being penetrated by signet ring cells, and also less frequently mixed with mucinous or tubular adenocarcinoma. The expression of E-cadherin protein is reduced or absent in the tumor cells, while it’s normal in adjacent non-neoplastic mucosa, by immunohistochemical test.
Individuals with a heterozygous germline CDH1 mutation have a lifetime risk of 70% in male and 56% in female of developing diffuse GC, and women with the CDH1 mutation were found to have a 42% lifetime risk for lobular-type breast cancer [3, 23]. According to Cisco et al. [24], four fifths of females and two thirds of males who are carriers of CDH1 gene mutations will develop HDGC by the age of 80 years. Individuals who met the HDGC diagnose checklist but did not have CDH1 mutations had longer survival times than GC cases harboring germline CDH1 mutations (48% vs. 36% survived for one year and 13% vs. 4% survived for five years) [3]. In mutation-positive patients, prophylactic total gastrectomy is recommended. With early detection (i.e., confined to mucosa and submucosa), >90% of patients with GC will be alive at 5 years compared with 10–20% of patients with advanced GC, even after potentially curative surgery has been carried out. Patients with a clinical diagnosis of HDGC could be tested for the CDH1 gene mutation. While the patients with the CDH1 gene mutation should be managed with more frequent endoscopic surveillance and biopsies, their children and/or relatives could be tested for the carrier status of CDH1 and therefore receive appropriate clinical management.
1.3 Gastric Adenocarcinoma and Proximal Polyposis Syndrome
GAPPS is a recently described autosomal dominant inherited GC syndrome with increased risk of gastric carcinoma, specialized with unique proximal gastric fundic gland polyposis (often less than1cm in size), with areas of multi-atypical hyperplasia lesions and subsequent intestinal-type GC formation (about 12.7% in call cases) [25, 26]. The typical gastric phenotype and the earliest GC has been observed at 10 and 33 years of age, respectively [25].
The diagnosis can be set up only after excluding the use of proton pump inhibitors (PPIs) and other heritable gastric polyposis syndromes, such as attenuated FAP, FAP, MAP, PJS, and CS. Though cases with Peutz-Jeghers syndrome may also carry fundic gastric polyps and excluding GC, the distribution of polyp is different from that in GAPPS [27, 28].
There is a diagnostic checklist which should be considered. Firstly, we count the number of polyps. For example, an index case with more than 100 gastric polyps carpeting the proximal stomach should be considered as GAPPS. The other situation is that a first-degree family of a known case carries over 30 polyps. Secondly, the location of polyps should be taken into account. In a case with no evidence of colorectal or duodenal polyposis, we may put it into GAPPS if his/her gastric polyps are restricted to the fundus and body of the stomach. Thirdly, histological feature should be thought over. If there are local areas presenting dysplasia or gastric adenocarcinoma in fundic gland polyposis (FGPs), the case would be diagnosed with GAPPS. The last but not the least, the autosomal dominant pattern of inheritance should be taken into consideration. Macroscopically, the lesions of GAPPS present florid and usually less than 1cm. The number of polyps distributing in the gastric fundus and body is more than 100, with relative less along the lesser curve of the stomach and without effecting gastric antrum and pylorus [25, 26]. Histologically, most of the lesions present fundic gastric polyposis without or with regions of atypical hyperplasia. Occasionally, adenomatous and hyperplastic polyps can be found with pure features or mixed characteristics focally within the fundic gastric polyps [27, 29]. The cases who met GAPPS would develop GC of intestinal type. The etiology and genetic cause of GAPPS with incomplete penetrance is unclear. In 2016, one study revealed that point mutations in APC promoter 1B were at risk of gastric adenocarcinoma in patients with GAPPS. The families with familial adenomatous polyposis may also harbor point mutations in APC promoter 1B [30]. However, mutations in APC, BMPR1A, CDH1, MUTYH, PTEN, SMAD4, and STK11 were preclusive in some families [26, 28].
1.4 Familial Intestinal Gastric Cancer
FIGC is an autosomal dominant inherited disorder; however, the genetic cause involved is currently unknown. It’s lacking the mutation of CDH1 and poorly characterized genetic predisposition for intestinal-type GC [5, 10].
The recommended diagnosis checklist varies in regions with different incidence of GC [31]. Diagnosis criteria similar to the Amsterdam criteria for colorectal carcinoma (CRC) have been applied in regions with high incidence. According to the Amsterdam criteria, only 0.9% cases (31/3632 families) in Japan met the diagnosis checklist for FIGC. 28.6% of patients develop GC younger than 50 years [4]. In regions with low incidence, guidelines include the following clinical criteria [4, 27]: (a) more than one case of GC in first-degree or second-degree relatives, with one or more confirmed patient of intestinal type in someone before the age of 50, and (b) at least three confirmed individuals of intestinal GC in first-degree or second-degree relatives, independent of age.
The GC display the common general features observed in the sporadic setting. Histologically, the tumors show the characteristics of Lauren intestinal-type adenocarcinoma [27]. Genetically, we do not find TP53, DNA mismatch repair genes, or CDH1 mutation in these tumors so far. However, some research reported that almost 17% of cases present epigenetic methylation of CDH1. There are also 9.4% of cases attracting attention because of loss of heterozygosity [31].
1.5 Lynch Syndrome
LS, also known as hereditary nonpolyposis colorectal cancer syndrome (HNPCC), is an autosomal dominant syndrome. About 2–4% of all CRC are LS, which is the most common form of inherited CRC syndrome [4]. Now we know there are two types of Lynch syndrome according to the clinical feature. The type, which is predisposing primarily to colonic carcinoma, we definite it as Lynch syndrome I. Part of tumors arising in the genitourinary tract, prostate, pancreaticobiliary tract, stomach, and endometrium have been identified as part of the neoplasm spectrum in Lynch syndrome II. In Lynch syndrome II, the lifetime risk of LS patients is up to 80% for CRC, 20–60% for endometrial cancer, and 11–19% for GC [32]. The lifetime risk for developing GC varies in different regions. In the Eastern, the lifetime risk of GC in LS patients (30% in Korea and 44.4% in China) is higher than it is in some Western countries (11–19%, even 2.1% in the Netherlands) [4, 32]. In Finland, the cumulative incidence of GC in LS is 13% by 70 years of age, and 52% of GC in LS are diagnosed in individuals younger than 50 years [33, 34]. GC associated with LS is predominantly intestinal phenotype, and the prevalence of Helicobacter pylori infection in LS patients with GC does not differ from it in the general population [35].
The predisposition of LS to cancers is related to the mutations of mismatch repair (MMR) genes, which would accelerate DNA microsatellite instability (MSI). Abnormal MMR gene can be found in 90% of tumors from LS patients with germline mutations and in 10–15% of sporadic cancers [36]. MMR proteins include the MutS proteins (such as hMSH2, hMSH3, and hMSH6) and the MutL proteins (such as hMLH1, hPMS1, hPMS2, and hMLH3) [37]. At least five MMR genes have been identified in LS, with approximately 90% of gene mutations in MLH1 and MSH2, 7–10% in MSH6, less 5% in PMS2, and very rarely in PMS1 [38]. The identification of MMR genetic alterations has a considerable clinical significance on the screening, diagnosis, and prevention of LS [32, 39]. MSI is a marker of the presence of replication errors in simple repetitive microsatellite sequences due to DNA MMR deficiency. Tumors are classified as microsatellite stable (MSS), MSI-low (MSI-L), and MSI-high (MSI-H). Several studies have reported that MSI is present in both familial and sporadic GC and that about 20–30% of GC have MSI [40]. MSI occurs at the stage of chronic gastritis, a long time before the development of GC [41]. Therefore, MSI analysis is promising as a valuable marker of the risk of progression to GC.
In the past, both Amsterdam criteria and Bethesda criteria have ever been used to establish a diagnosis of LS. GC, however, is not a defining criterion for LS in either classification. In 2004, the revised Bethesda guidelines, instead of the Amsterdam I and II, was proposed as the clinical screening criteria that can be used to select individuals for MSI analysis. Firstly, the patients, who was diagnosed with CRC at less than 50 years of age, are suspected to have LS. Secondly, we take the cases, which meet the criteria that present synchronous, metachronous colorectal, or other LS-related tumors at any age, into account. Thirdly, we think over the individuals who suffer from CRC diagnosed before the age of 60 years, and its MSI phenotype was high. Fourthly, CRC patients, whose first-degree family member was diagnosed with a LS-related tumor at less than 50 years of age, should be taken into consideration or CRC patients, who has family history and the relative diagnosed with a LS-related tumor at any age. If a patient fulfills the above diagnosis checklist, the molecular (such as PCR and direct DNA sequencing) and/or immunohistochemical (MMR protein) testing for MSI should be performed, because certain cases may fulfill the clinical feature but are MMS on testing, an exclusionary feature [42]. The presence of MSI in a tumor specimen is not indicative of a particular gene defect, and neither MSI nor IHC can distinguish between sporadic and LS-related cancer. However, IHC results indicating the absence of a specific MMR protein can be used to determine which targeted mutation analysis should be performed [43]. A full-scale analysis of the entire MSH6, PMS2, MSH2, and MLH1 genes is commendatory for making a definite diagnosis of LS [4]. The most common abnormity is seen in MSH2 (about 60% of LS cases) and MLH1 (about 30% of the cases). The remaining rare types are PMS2, MSH6, TGFBR2, and MLH3 mutations (about 10% of the cases) [4]. Some method for MSI detection in GC has been proposed, but whether it will become the treatment standard remains unknown [44]. In addition, the relative rarity of GC in LS families makes the cost-effectiveness of endoscopic screening questionable [4].
1.6 Familial Adenomatous Polyposis/MUTYH-Associated Polyposis/Attenuated Familial Adenomatous Polyposis
FAP is an autosomal dominant disease classically characterized by hundreds to thousands of adenomas through the gastrointestinal tract. Its etiology is adenomatous polyposis coli (APC) germline mutation which is located on chromosome 5q21. There are three clinical features that help us diagnose FAP. We take the cases, which can be detected in more than 100 adenomatous colorectal polyps, into account. APC germline mutation may also be a clue. If young people, whose first-degree or second-degree family member was diagnosed with FAP, carry any number of adenomas, he/she would be considered as FAP. Nearly 8% of FAP cases are attenuated FAP (AFAP), which characteristic is less than100 adenomatous colorectal polyps. Usually these patients carry 10–99 adenomas at age older than 30 years and have one first-degree family member with CRC and few adenomas. The other situation is two or more relative with 10–99 adenomas at age older than 30 years old [45]. MAP is an autosomal recessive polyposis syndrome [37]. A diagnosis is established only after exclusion of FAP syndrome by demonstrating an absence of APC mutation and confirmation of the biallelic mutations of MUTYH gene, a mut Y homolog (Escherichia coli) gene located at chromosome loci 1p34.3-p32.1, in a suspected individual on the basis of the given circumstances. Firstly, we take the cases, whose family member was diagnosed with CRC accordingly with an autosomal recessive pattern of inheritance, into account. Secondly, those who can’t be detected in the germline mutation of APC gene and carry more than 100 colon polyps would be thought over. The third situation is those who harbor 10–100 colon polyps, whatever adenomas or hyperplastic type. Fourthly, we would take an individual who carries 1–10 colon adenomas in the first decade into consideration. The fifth part includes the cases with specific somatic mutation of KRAS (c.34G→T) in codon 12 and suffering from CRC at the same time. Extra-colonic neoplasms are observed often in patients with FAP, but clinical features of GC associated with FAP are not clear at present. The presence of gastric polyps (from 51% to 88% in FAP [46, 47] and 93% in AFAP [48]) and even polyps associated with dysplasia or canceration is the known manifestation of FAP/AFAP in the Eastern [4]. The risk of GC in FAP varies in different regions. A high risk has been reported in the Eastern (3.8% in Japan [49] and 4.2% in Korea [50]) but low in the Western (0.6%) [51], and GC related to FAP often originated from fundic gland polyps or adenomatous polyps. Patients with FAP are 7–10 times more likely to affect gastric carcinoma than nonsyndromic patients in the Eastern [52]. For example, in Japan, FGP were significantly more common in FAP than in AFAP; however, GC was significantly less common in FAP than in AFAP. Upper gastrointestinal tumors/polyps were frequently found in patients with FAP, but the frequency of GC in patients with FAP was similar to that in the general population [49]. The age of onset of stomach manifestations is variable, but GC typically develops long after colectomy. The types of benign gastric lesions detected include FGPS, GAs, and, infrequently, hyperplastic polyps and pyloric adenomas. Syndromic FGPs have a higher incidence of carrying beginning dysplasia (25–44%) than sporadic cases (~1%) [53, 54]. The dysplasia often present low grade, and the risk of malignant transformation is low. Gastric involvement in patients with MAP is uncommon. Although gastric lesions, such as adenomas and fundic gland polyps, have been found in up to 11% of patients with MAP, the risk of GC does not increase currently [55].
The FAP syndromes are autosomal dominant inherited disorders with a close to 100% penetrance. The involved gene is the tumor suppressor gene of adenomatous polyposis coli (APC) located on chromosomal 5q21, which harbor heterozygous mutation. About 90% of germline inactivation of APC lead to truncation of APC protein. APC mutations have been proved to be associated with some gastric lesions, such as gastric fundic gland polyposis, gastric adenomas, and dysplastic and malignant gastric polyps. MAP is an autosomal recessive polyposis syndrome caused by the MUTYH gene located on 1p34.1, which plays a significant role in DNA base-excision repair.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree