© Springer Science+Business Media New York 2015
Ashraf Khan, Ian O. Ellis, Andrew M. Hanby, Ediz F. Cosar, Emad A. Rakha and Dina Kandil (eds.)Precision Molecular Pathology of Breast CancerMolecular Pathology Library1010.1007/978-1-4939-2886-6_1616. Molecular Pathology of Breast Cancer Metastasis
(1)
Department of Histopathology, Division of Cancer and Stem Cells, School of Medicine, The University of Nottingham and Nottingham University Hospitals NHS Trust, Nottingham City Hospital, Nottingham, NG5 1PB, UK
Keywords
Breast cancerMetastasisVascular invasionMolecular featuresIntroduction
Breast cancer development and progression follow the known seven fundamental changes in cellular physiology, which together determine the malignant phenotype known as the ‘Hallmarks of Cancer’. These hallmarks are: self-sufficiency in growth signals, insensitivity to growth inhibitory signals, limitless replicative potential, evasion of apoptosis, defective DNA repair, sustained angiogenesis, and the ability to invade and metastasise [1, 2]. Although malignant solid tumours, including breast cancer, arise from clonal expansion of a single transformed cell, the transformed mass of cells becomes enriched with some variants/clones more able to evade host defences and is likely to be more aggressive [3].
Metastasis, the ability to invade and metastasise, is formation of tumour implants discontinuous with the primary tumour. This occurs through colonisation of remote or distant organs by malignant cells having the privilege to invade and metastasise.
Metastasis is the crucial unequivocal difference between a tumour being benign, locally malignant or malignant. In stricter terms, the capacity of at least some of a tumour cell population to detach, migrate and colonise remote tissues is a “sure” distinctive feature of malignant tumours from benign and locally malignant. However, not all detached invasive malignant cells succeed in completing the stressful journey from their primary location to colonise the remote organ. Some cancer patients may remain metastasis-free for long times, and even some of them may never develop metastatic deposits [4, 5]. The morbidity and mortality in breast cancer patients are mainly attributable to development of loco-regional or systemic spread of cancer or recurrences of both [6, 7]. Metastases are the cause of 90 % of human cancer deaths [8].
It is noteworthy that breast cancer evolves from a pre-invasive lesion or carcinoma in situ; in which cells display all cytological features of malignancy without invasion of the basement membrane, to an overtly invasive stage. The latter is considered one step removed from invasive cancer; and with time, cells penetrate the basement membrane, infiltrate the sub-epithelial stroma and hereby could metastasise to remote organs [9, 10].
The process of metastasis is collectively known as the metastatic cascade, during which a number of sequential steps have to be completed by cancer cells in order to successfully establish a metastatic focus at a distant location. This process requires the integration of complex arrays of specific molecular pathways. These, from theoretical standpoint, could be divided into the following categories:
Prerequisites for Metastasis
For malignant cells to metastasise, they must fulfil certain tumourigenic functions that are considered as fundamental prerequisites for metastasis. These include: unlimited cellular proliferation, evasion of cell intrinsic and environmental constraints, attraction of a blood supply; angiogenesis, and gaining the capacity to detach, migrate and move away from their original locations. Although such functions are acquired early during tumour initiation and local development and are considered amongst the hallmarks of cancer, they must remain dynamically active throughout malignant progression. The acquisition of these functions is cumulatively gained through tumour-initiating mutations, genetic alterations that occur secondarily to genomic instability and epigenetic changes. With progressive tumour growth, tumour must selectively overcome environmental stresses including cytotoxic immunity, diminished oxygen supply and an acidic environment resulting from enhanced cellular metabolism. Therefore, having these features of aggressiveness is considered prerequisites for metastasis, without which cells would not possess the capacity to proceed into the next metastatic phases [11, 12].
Metastatic Initiation and Dissemination
Dissemination commences when aggressive tumour cells become locally invasive and readily get access into the bloodstream through the newly formed vasculature, which is typically pathologically leaky due to its wide fenestrations, leading to easier entry into the circulation [13]. Moreover, such intravasation is enhanced by an epithelial-to-mesenchymal transition programme, proposed genetic reprogramming of carcinoma cells that confers superadded motility to these cells [14]. In other terms, malignant cells are continually shed from the parent primary tumour mass and circulate within the blood stream. The rate of malignant cell shedding generally increases with increased tumour size [15].
Epithelial Mesenchymal Transition
Mammary epithelial cells are functionally and phenotypically different from the supporting mesenchymal cells. The former form highly specialised surface joined together by specialised junctions and rest on well-organised basal lamina. In addition to basal contact between epithelial cells and basal lamina, epithelial cells have apical-basolateral polarisation that is tightly controlled through localised distribution of adhesion molecules and cellular cytoskeleton [16, 17]. Mesenchymal cells, on the other hand, do not exhibit such architectural organisation, contact each other only focally and lack an underlying basal lamina, which might facilitate their propensity to migrate [18].
The term epithelial–mesenchymal transition (EMT) describes a sequence of events during which epithelial cells lose many of their epithelial characteristics and gradually gain those typical of mesenchymal cells [19]. EMT phenomenon involves complex changes in cell architecture and behaviour and encompasses a wide spectrum of inter-cellular and intra-cellular changes, which are probably controlled by diverse extracellular signals [20]. Loss of epithelial characteristics and gain of mesenchymal characteristics confer invasive and migratory capacity to the malignant cells. However, it is widely conceived that EMT is proposed to be a transient reversible process [14]. Nevertheless, it is noteworthy that EMT does not necessarily denote a lineage switch; but rather a series of complex changes manifesting through phenotypic and functional alterations of malignant cells [21, 22].
EMT has been described with much emphasis in tissue culture and animal models using cancer cell lines [23, 24]. The manifestation of EMT in tissue cultures prompted some authorities to describe EMT as a mere cell culture phenomenon that lacks direct clinical evidence or clear molecular markers in breast cancer [25]. For instance, under specific culture conditions, epithelial cells can transform into fibroblast-like cells, whereby cells could not attain epithelial type polarity; aided by the culture conditions that facilitate discohesiveness. Moreover, cytokeratins commonly used as molecular identifiers of carcinoma cells are often downregulated, while vimentin is upregulated; leading ultimately to difficult characterisation of cultured epithelial cells undergoing EMT from the surrounding stromal cells [26–28].
Whether EMT is a prerequisite of tumour progression or not is a controversial matter. For instance, EMT has been considered as a key role-player in cancer dissemination from local to remote sites [29]. On the other hand, others report that EMT is one possible mechanism behind the process of local invasiveness and progression of cancer [24, 30]; others [31] still doubt its occurrence in real cancers. In addition, breast cancer progression without EMT has been reported [32]. However, Giampieri and colleagues in their recent works demonstrated the impact of transient TGFß signalling; an EMT trigger, in switching cancer cells from cohesive “collective” to “single cell” motility, which is essential for blood-borne metastasis [33].
Recent insights into molecular EMT-controlling mechanisms indicate that several complex signalling pathways are involved in the initiation and execution of EMT in the contexts of development and cancer progression. These molecular mechanisms significantly overlap with those controlling cellular adhesion, motility, invasion, survival and differentiation [20, 34]. A number of specific molecular pathways and transcription factors have been reported as ‘‘EMT triggers’’ including TGFß [35], Twist1/2 [36], PI3 K/Akt pathway [37], CTEN [38], Snail, Slug, and Zeb1 [39]. When expressed in a variety of cell types, these factors act as transcriptional repressors of E-cadherin and alter the expression of a diverse number of genes denoting in vitro EMT with subsequent promotion of cancer invasion and metastasis [38, 40].
Not only is E-cadherin downregulation considered as the sole characteristic molecular phenotypic change of carcinoma cells reported to accompany breast cancer metastasis through EMT programme but upregulation of N-cadherin or “cadherin switching” has been reported to enhance cellular motility of mammary epithelial cell lines [41]. Moreover, N-cadherin expression was reported to be more influential than E-cadherin loss in determining the EMT phenotype and hence progression [42]. The latter notion that E-cadherin loss alone is not sufficient for breast cancer metastatic dissemination is supported by the known character of invasive lobular carcinoma which is typically characterised by loss of E-cadherin expression [43].
Therefore, these integrative molecular changes challenge some prevailing views that propose repression of E-cadherin or deregulation of a single molecular pathway to be sufficient to explain the tendency of certain cancer types to disseminate over others [38, 44].
Furthermore, the privileged occurrence of EMT in some particular breast cancer molecular subtypes has been reported [45]. Different breast cancer molecular subtypes express differential levels of cadherin family members; E-cadherin, N-cadherin and P-cadherin, and upregulation of mesenchymal markers (e.g. SMA); with N-cadherin gain having the highest influence in determining the EMT phenotype [46].
The Tumour Microenvironment and Metastasis
The tumour microenvironment in invasive breast carcinoma normally represents mechano-biological interface between malignant cells and the components of the surrounding stroma [47]. There are different types and subtypes of cells contributing as pro-metastatic or anti-metastatic regulators.
Fibroblasts are spindly, non-epithelial, non-endothelial and non-inflammatory stromal cells. Their main biological functions in normal tissues are the regulation of epithelial cells differentiation, wound healing, secretion of variety collagens types and they are the main producers of ECM proteases [48]. The close presence of malignant cells and the stromal fibroblast in the same local microenvironment will lead to epigenetic alteration of the latter, where some of the genes regulating cellular proliferation of normal fibroblasts may be affected [49]. This paracrine signalling loop may mutate or silence well-known tumour suppressor genes such as P53 and PTEN within cancer associated fibroblasts (CAFs) [50]. CAFs are obligated by their intrinsic genetics and the epigenetic effects of the adjacent malignant cells to secrete growth factors, chemokines, cytokine, and ECM remodelling enzymes [48]. It also may supress the function of immune T cells [51]. Moreover, CAFs and inflammatory cells promote the upregulation of the transcriptional factors genes SNAIL, SLUG, and ZEB1, factors reported as EMT triggers [39]. Probably one of the fascinating examples of synergism between CAFs and breast cancer cells is podoplanin; a glycoprotein encoded by the PDPN gene and expressed in lymphatic vessels, malignant cells and CAFs. It functions as a booster of cellular motility and reducer of cellular adhesion. Overexpression of podoplanin is significantly correlated with breast cancer aggressiveness. Interestingly, podoplanin overexpression in the invasive front of breast carcinoma is the back-up plan of invasion and metastasis if the pathways of EMT are inhibited or hindered, through remodelling the cytoskeletal structures and forming invasive protrusions: the filopodia [52]. Therefore, this phenomenon illustrates the potent synergism between paracrine signalling from CAFs and epigenetics from malignant cells to invade lymphatic vessels.
Different phenotypes of lymphocytes and myeloid cells are present in the tumour microenvironment. The presence of T lymphocytes is reported to be associated with good prognosis in the case of Cytotoxic CD8+ memory T cells and CD4+ T helper 1 cells [53]. Cytotoxic CD8+ memory T cells are able to eradicate malignant cells at the invasive front of the tumour. This tumour-cell cytotoxicity is supported by CD4+ T helper 1 cell cytokines; Interleukin-2 (IL-2) and Interferon gamma (IFN-γ), which play crucial roles in the differentiation of naive T cells into memory T cells. The latter cells can fight tumour formation with cytostatic and cytotoxic mechanisms [54]. In contrast to Cytotoxic CD8+ memory T cells, Natural Killer (NK) and Natural Killer T (NKT) cells infiltrate the tumour stroma with no major effects on malignant cells [53].
Although infiltrating CD4+ cells had good prognosis implications, not all the CD4+ cells subtypes possess same influence. For instance, T regulatory cells (Tregs) have immune-inhibitory characteristics with subsequent protection of malignant cells from cytotoxic CD8+ T cells’ elimination [55]. This opposition of the immune function could be attributed to the suppression of the cellular recognition of the malignant cells due to the expression of CD152 receptor protein (also known as cytotoxic lymphocyte associated protein-4, CTLA4) that reverses the activation of the immune response and transmits inhibition signals. Moreover, the immune dysregulatory actions of transcriptional factors forkhead box P3 (FOXP3) and others are other explanations [56, 57].
The other type of lymphocytes of the adaptive immune system is B lymphocytes. Generally speaking, B lymphocytes have good prognostic implications if they infiltrate the tumour microenvironment owing to production of specific antibodies against the tumour cell antigens [58]. However, the vast majority of those immunity cells tend to localise further in the stroma or even at lymphoid structures contiguous to the microenvironment, contrasting cytotoxic CD8+ T cells that are often proximate to the malignant cells [59]. Likewise the inhibitory effect of Tregs, regulatory B cells (Bregs) impairs the tumour specific immune response. Furthermore, it induces a special immune evasion mechanism on behalf of the malignant cells [60].
In contrast to the tumour infiltrating lymphocytes, tumour associated macrophages (TAMs) are strongly linked with breast cancer poor prognosis. TAMs are well-known to produce Interleukin-10 and angiogenic factors [61], and they aggregate nearby necrotic and hypoxic areas of the tumour microenvironment [62]. Hence, and under this hypoxic environment, growth factors that promote blood and lymph neovascularization are upregulated and secreted by the malignant cells. Vascular Endothelial Growth Factor-A (VEGFA) is essential requirement for hemangiogenesis, and it is a chemoattractant for macrophages [63]. The attracted and recruited macrophages will aid the invasion and metastasis process through a dual-direction reaction loop of paracrine signalling with the malignant epithelial cells. Therefore, the intravasation of malignant cells into BV or LV and their migration through endothelium will be facilitated [64]. Moreover, this may lead to suppression of cytotoxic T cells and increase the propagation of pro-metastatic phenotypes of TAMs [65]. Therefore, the use of immunomodulation drug therapy aims to interfere with the tumour-induced immunosuppression mechanisms, and it is more likely to be beneficial than using non-specific cytotoxic drugs [66].
Invasion of the Extracellular Matrix (ECM)
The ECM is the complex structural entity surrounding and supporting mammary epithelial cells and is physically defined into the basement membrane and the interstitial tissue. Three major classes of macromolecules are physically associated to form the ECM; structural proteins, assembled into fibrous components, such as collagens and elastins, a diverse group of adhesive specialised glycoproteins, including fibrillin, fibronectin and laminin and a gel of proteoglycans and hyaluronan [67]. Four different families of collagen are characterised, where type I, II and III are the most abundant and form fibrils of similar structure. Type IV collagen forms a two-dimensional reticulum and is a major component of the basement membranes, and in such a case it is synthesised by epithelial and endothelial cells [68]. The ECM not only provides physico-mechanical and structural support for the mammary epithelium yet it encodes a large variety of specific signals which dynamically influence breast cancer cell growth, invasion and migration [69, 70].
Following detachment from the primary tumour cell mass, the breast carcinoma cells first adhere to the matrix components. Receptor-mediated attachment of the malignant cells to laminins and fibronectin is critically important for invasion and metastasis [71–73]. Within the interstitial ECM, breast carcinoma cells must create their passageway for migration through active proteolytic degradation of the ECM components. Such enzymatic degradation is carried out by proteases at the invading edge of the tumour [74]. Different classes of proteases have been evidenced as pro-metastatic proteins including the serine [75], cysteine [76], aspartic [77] and the matrix metalloproteinases (MMPs) [78]. The latter is a broad family of zinc-dependant endopeptidases comprising 23 members in humans. To date, at least fourteen MMPs are implicated in breast cancer development and progression [79]. MMPs are produced both by the malignant cells and the peritumoural stromal [80]. The action of proteases is tightly regulated by antiproteases which increase in response to elevated protease level. It is the balance between proteases and antiproteases at the invading/cutting edge of the tumour which promotes or halts tumour cell propagation [79, 81]. Proteolysis of ECM facilitates breast cancer cell invasion through their propulsion, or simply, locomotion. Migration is an active process and is affected by multiple factors, both autocrine and tumour-cell derived motility factors, and cleavage products of matrix components derived from collagen and proteoglycans [82, 83]. Cells migrate through the ECM by extending membrane protrusions; filopodia and lamellipodia, at their leading edges. Their formation is driven by the reorganisation and polymerisation of actin filaments. The propagation of tumour cells is a complex highly integrated process controlled by complex signalling cascades within the cells and cell-stromal crosstalk [83, 84].
Lympho-Vascular Invasion
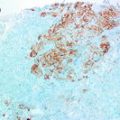
Lymphatic Vascular Invasion (LVI) is one of the crucial steps in the metastatic cascade. Presence of LVI, as detected by histopathological examination in invasive breast cancer, is a marker of metastatic potential, a predictive factor of metastasis into the lymphatic system and is associated with poor outcome [85]. In a previous study of 3812 invasive breast cancer we have demonstrated that LVI is strongly associated with outcome in the entire series and in different subgroups. LVI was an independent predictor of outcome and provided survival disadvantage equivalent to that provided by 1 or 2 involved lymph nodes and to that provided by 1 size category [85]. Using a panel of endothelial markers in more than 1000 invasive breast cancer we [86] have demonstrated that VI was present 22 and in 97 % of cases it was LVI. Blood vascular invasion (BVI) was detected in 3 % of cases [86]. The current clinicopathological studies that investigated the role of LVI in breast carcinoma focused on the capacity, the size, and the location of the vessel involved; whether intratumoural, peritumoural, or both [85, 87]. Immunohistochemical detection of LVI and BVI improves their detection rate and prognostic/predictive values in primary invasive breast cancer [86, 88, 89].
The multiple chemotaxis and cytotaxis interactions due to the EMT and their consequences in the tumour microenvironment will dramatically alter the adjacent lymphatics [90]. The angiogenesis and lymphangiogenesis are strongly related with tumour aggressiveness. The presence of BV and LV within the solid tumours is an eminent sign of the stimulation of embryogenesis of the endothelium by the malignant cells. Complex cellular events, including proliferation, sprouting, migration and tube formation are involved in the process of lymphangiogenesis. These functions depend on vascular endothelial growth factor receptor 2 (VEGFR2) and VEGFR3 signalling controlled by VEGFC or VEGFD [91]. These signalling events are more or less the counterparts of the molecular regulation of angiogenesis by VEGFA signalling via VEGFR2 [92].
The intratumoural vessels usually get collapsed under the increased mass of the growing tumour [93]. On the other hand, the peritumoural vessels, and under the influence of the VEGFC and VEGFD, are highly proliferative. The enlarged peritumoural vessels have been detected with substantial diameter that lead to an increase of the contacting surface area between malignant cells and LV, which indicates higher propensity of the LVI incidence [94]. Indeed, the chemotactic and cytotactic effects are crucial in tumour metastasis; nevertheless, the transcellular lymphatic drainage that is caused by the elevated interstitial flow of lymphatic fluids may polarise the malignant cells toward the nearest LV through the autologous secretion of C-C Chemokine Receptor 7 (CCR7). This expression of the specific receptor and its specific ligand portrays the physio-chemical autocrine mechanisms possessed by some clones of the malignant cells [95]. The latter is an example illustrating the capacity of some malignant cell to intensify the chemokinetics of metastatic progression via directing themselves to the preferential metastasis target.
Recently, the significant association between the breast cancer molecular subtype and LVI and LN metastasis has been documented through the clinical trial performed by American College of Surgeons Oncology Group (ACOSOG) [96]. Triple negative or basal-like molecular subtype showed the least association with LVI and axillary LN metastasis although it is strongly related with poor prognosis and high propensity to recur locally. Conversely, the Estrogen Receptor and Progesterone Receptor and HER2 positive molecular subtypes showed the highest incidence of LVI. Triple negative malignant cells are more mitotic [97], and less likely to express claudin protein than the positive ER, PR, and HER2/neu molecular subtypes. Furthermore, the triple negative molecular subtype expresses proteins that are crucial to transform the malignant epithelial cell to the mesenchymal form; hence, are potently invasive and metastatic [98]. These results are reinforced by the recent report from the Danish Breast Cancer Cooperative Group reporting reduced risk of axillary lymph node involvement at the time of diagnosis in triple negative breast cancer compared to patients with other subtypes [99]. Therefore, tumour invasiveness and the routes through which breast cancer disseminates to regional lymph nodes or distant metastatic sites are different among to the known breast cancer molecular subtypes.
Following invasion into the local stromal and intravasation, the malignant cells must manage to survive within the luminal cavities of the LV or capillaries [100]. To survive the stresses during their journey, malignant breast cancer cells require intrinsic functions, as for instance evading detachment-triggered cell death through AKT signalling pathway activation via neurotrophic tyrosine kinase, receptor type 2 (NTRK2, TrkB) [101]. Moreover, cells must withstand the associated extrinsic shear forces and platelet aggregates [102]. They also need to overcome and escape phagocytosis by circulating macrophages. A subset of circulating luminal breast cancer cells has been reported to express CD47 molecule that helps tumour cells evade macrophage phagocytosis [103].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree