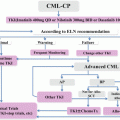
Mechanism of resistance
Treatment strategies
BCR-ABL1-dependent
ABL1-kinase domain mutationsa
Second-generation TKIs
BCR–ABL1 amplification
BCR-ABL1-independent (intrinsic)
Activation of downstream signalling
JAK-STAT pathway
PI3K/AKT/mTOR pathway
JAK2 inhibitors (ruxolitinib) with TKIs [58]
Genetic polymorphismsb
BH3 mimetics with TKIs [49]
Drug transport pumpsb
Epigenetic modificationb
HDAC inhibitors (panobinostat) [82]
miRNA-targeted therapy
Persistence of LSCs
Wnt/β-catenin pathway
Hedgehog pathway
Arsenic trioxide
Cyclooxygenase inhibitors (indomethacin) [87]
BCR-ABL1-independent (extrinsic)
Bone marrow environment
Cytokines
Hypoxia
Anti-HIF1 [76]
11.3.1 Tackling ABL1-Kinase Mutants
Second-generation TKIs, such as dasatinib and nilotinib, have been approved for first-line therapy for CML as well as second-line therapy for those with imatinib failure [5]. Dasatinib, a dual Src/ABL1-kinase inhibitor, is active against many imatinib-resistant mutants except the T315I [8, 90]. Nilotinib, with N-methylpiperazine moiety incorporated into imatinib, is a more potent compound against most of the clinically relevant BCR–ABL1 mutants, except the T315I mutant [8, 90, 91].
Ponatinib is a pan-TKI effective against the T315I mutation and has recently been approved for the treatment of patients who failed previous TKI therapy [5, 77, 78]. However, its use has to be carefully monitored against its toxicity since serious cardiovascular, cerebrovascular and peripheral vascular adverse events have been reported in the study, especially in the presence of risk factors including hypertension, hyperlipidaemia and diabetes [99].
Allosteric inhibitors, such as ABL-001, which target sites on BCR-ABL1 other than the ATP-binding site, have been shown to prevent TKI resistance in preclinical models, and the results of ongoing phase I studies are eagerly awaited [www.clinicaltrials.gov identifier: NCT02081378].
11.3.2 Targeting Downstream Signalling Pathways
Combining TKIs with compounds targeted against different downstream signalling pathways has been widely studied in preclinical and clinical studies [57]. JAK2 inhibitors induced apoptosis and decreased survival of CML stem cells in cultures and mouse models, when given together with imatinib [58, 93]. Early-phase clinical trials of ruxolitinib in combination with TKIs in CML with residual disease have been carried out.
Another approach is to combine mammalian target of rapamycin (mTOR) inhibitors with TKIs in treating TKI-resistant mutants [79]. Rapamycin has been shown to be effective in overcoming imatinib resistance in cell lines with BCR-ABL1 amplification or ABL1-kinase mutation [40, 80, 81, 100]. Allosteric mTOR inhibitors, everolimus and temsirolimus, have also been studied in phase 1 trials for CML.
11.3.3 Modifying Epigenetic Regulators
Histone deacetylase inhibitors (HDACi), such as suberoylanilide hydroxamic acid (SAHA), suppress BCR–ABL1 levels in TKI-resistant CML cells in vitro and in vivo [94, 95]. Panobinostat (LBH589) has recently been shown to eliminate quiescent CML LSCs that are otherwise resistant to elimination by imatinib in mouse models. Clinical studies of panobinostat in combination with imatinib are being carried out in CML patients with residual disease [82].
DNA methylation inhibitors (DNMTi) or demethylating agents have also been tested in CML patients with TKI resistance. Early clinical studies of decitabine treatment showed modest response in CML patients who developed resistance to imatinib but are now being tested in combination with TKIs [101, 102].
Therapy based on microRNA (miRNA) may also be another promising epigenetic approach as BCR–ABL1 regulates miRNA profiles and miRNA has targets on downstream signalling pathways in CML, such as PI3K/AKT/mTOR pathway [103].
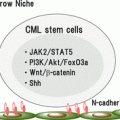
11.3.4 Eradicating CML Stem Cells
Wnt/β-catenin pathway can be an attractive novel target for the elimination of CML stem cells [87, 96]. A study in mice showed that indomethacin, a cyclooxygenase inhibitor, reduced β-catenin and inhibited proliferation of LSCs. The hedgehog pathway is another potential target since smoothened (SMO) antagonists are found to effectively decrease replication in CML cell lines in vitro [88, 89].
An alternative approach is the use of arsenic trioxide, which impairs BCR-ABL1 stability and causes depletion of CML LSCs through autophagosomal degradation [104, 105]. Early-phase clinical trials with those small molecules in combination with TKIs for resistant CML patients are being carried out [www.clinicaltrials.gov identifier: NCT00250042 and NCT01397734].
11.3.5 Targeting Bone Marrow Environment and Cytokines
Modification of inflammatory cytokines, leukotrienes and prostaglandins by targeting against lipoxygenase and cyclooxygenase pathways in bone marrow environment has been shown to be a feasible approach to suppress proliferation and induces apoptosis of human CML cells and mouse models [73, 74].
Since hypoxia and up-regulated HIF1a in bone marrow environment have been implicated in TKI resistance, targeting HIF1a and associated genes may be a potential strategy to overcome resistance and eliminate CML LSCs [76].
11.3.6 Inducing Apoptosis and Inhibiting Autophagy
HSP90, a chaperone molecule for oncoproteins such as BCR-ABL1 can be inhibited by 17-allylamino-17-demethoxygeldanamycin (17-AAG) or tanespimycin [85, 86]. While studies using tanespimycin have been initiated, results of these studies are not publicly available.
Another potential therapeutic approach is inhibition of autophagy. Autophagy is a mechanism used by cancer cells to resist apoptosis and is characterized by intracellular formation of autophagosomes and breakdown/recycling of cell components [106]. CML cells can undergo autophagy to avoid apoptosis induced by TKIs and develop resistance to treatment. Compounds such as chloroquine and clarithromycin inhibit essential autophagy and increase the sensitivity of CML stem cells to treatment with TKIs [83, 84, 107].
11.4 Summary
With the advent of TKIs, patients with CML now enjoy increased survival with a reasonable quality of life. However, most patients will likely have to remain on long-term therapy with imatinib and second-generation TKIs. Even among those who achieve durable molecular response with imatinib, cessation of therapy results in recurrent disease in a significant majority. Accordingly, the outcomes of similar studies employing second-generation TKIs are underway.
The next lap of CML research will be directed at eliminating the CML LSCs and providing effective cure for CML off any drug therapy. With the current knowledge and understanding of CML pathogenesis, several targetable pathways have been identified. In practical terms, such studies will have to balance the possibility of long-term cure against toxicity.
References
1.
Hochhaus A. Educational session: managing chronic myeloid leukemia as a chronic disease. Hematology Am Soc Hematol Educ Program. 2011;2011:128–35.PubMed
2.
Hochhaus A, O’Brien SG, Guilhot F, Druker BJ, Branford S, Foroni L, et al. Six-year follow-up of patients receiving imatinib for the first-line treatment of chronic myeloid leukemia. Leukemia. 2009;23(6):1054–61.PubMed
3.
Deininger M, O’Brien SG, Guilhot F, Goldman JM, Hochhaus A, Hughes TP, et al. International randomized study of interferon Vs STI571 (IRIS) 8-year follow up: sustained survival and low risk for progression or events in patients with newly diagnosed chronic myeloid leukemia in chronic phase (CML-CP) treated with imatinib [abstract]. Blood (ASH Annual Meeting Abstracts). 2009;114:1126.
4.
Druker BJ, Guilhot F, O’Brien SG, Gathmann I, Kantarjian H, Gattermann N, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355(23):2408–17.PubMed
5.
Baccarani M, Deininger MW, Rosti G, Hochhaus A, Soverini S, Apperley JF, et al. European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood. 2013;122(6):872–84.PubMed
6.
Marin D, Ibrahim AR, Lucas C, Gerrard G, Wang L, Szydlo RM, et al. Assessment of BCR-ABL1 transcript levels at 3 months is the only requirement for predicting outcome for patients with chronic myeloid leukemia treated with tyrosine kinase inhibitors. J Clin Oncol. 2012;30(3):232–8.PubMed
7.
Branford S, Kim DW, Soverini S, Haque A, Shou Y, Woodman RC, et al. Initial molecular response at 3 months may predict both response and event-free survival at 24 months in imatinib-resistant or -intolerant patients with Philadelphia chromosome-positive chronic myeloid leukemia in chronic phase treated with nilotinib. J Clin Oncol. 2012;30(35):4323–9.PubMed
8.
Melo JV, Chuah C. Resistance to imatinib mesylate in chronic myeloid leukaemia. Cancer Lett. 2007;249(2):121–32.PubMed
9.
Milojkovic D, Apperley J. Mechanisms of resistance to imatinib and second-generation tyrosine inhibitors in chronic myeloid leukemia. Clin Cancer Res. 2009;15(24):7519–27.PubMed
10.
Ng KP, Hillmer AM, Chuah CTH, Juan WC, Ko TK, Teo ASM, et al. A common BIM deletion polymorphism mediates intrinsic resistance and inferior responses to tyrosine kinase inhibitors in cancer. Nat Med. 2012;18(4):521–8.PubMed
11.
Mahon FX, Deininger MW, Schultheis B, Chabrol J, Reiffers J, Goldman JM, et al. Selection and characterization of BCR-ABL positive cell lines with differential sensitivity to the tyrosine kinase inhibitor STI571: diverse mechanisms of resistance. Blood. 2000;96(3):1070–9.PubMed
12.
Gorre ME, Mohammed M, Ellwood K, Hsu N, Paquette R, Rao PN, et al. Clinical resistance to STI-571 cancer therapy caused by BCR-ABL gene mutation or amplification. Science. 2001;293(5531):876–80.PubMed
13.
Branford S, Rudzki Z, Walsh S, Grigg A, Arthur C, Taylor K, et al. High frequency of point mutations clustered within the adenosine triphosphate-binding region of BCR/ABL in patients with chronic myeloid leukemia or Ph-positive acute lymphoblastic leukemia who develop imatinib (STI571) resistance. Blood. 2002;99(9):3472–5.PubMed
14.
Roche-Lestienne C, Soenen-Cornu V, Grardel-Duflos N, Laï JL, Philippe N, Facon T, et al. Several types of mutations of the Abl gene can be found in chronic myeloid leukemia patients resistant to STI571, and they can pre-exist to the onset of treatment. Blood. 2002;100(3):1014–8.PubMed
15.
Roumiantsev S, Shah NP, Gorre ME, Nicoll J, Brasher BB, Sawyers CL, et al. Clinical resistance to the kinase inhibitor STI-571 in chronic myeloid leukemia by mutation of Tyr-253 in the Abl kinase domain P-loop. Proc Natl Acad Sci U S A. 2002;99(16):10700–5.PubMedCentralPubMed
16.
Shah NP, Nicoll JM, Nagar B, Gorre ME, Paquette RL, Kuriyan J, et al. Multiple BCR-ABL kinase domain mutations confer polyclonal resistance to the tyrosine kinase inhibitor imatinib (STI571) in chronic phase and blast crisis chronic myeloid leukemia. Cancer Cell. 2002;2(2):117–25.PubMed
17.
Corbin AS, La Rosée P, Stoffregen EP, Druker BJ, Deininger MW. Several Bcr-Abl kinase domain mutants associated with imatinib mesylate resistance remain sensitive to imatinib. Blood. 2003;101(11):4611–4.PubMed
18.
von Bubnoff N, Schneller F, Peschel C, Duyster J. BCR-ABL gene mutations in relation to clinical resistance of Philadelphia-chromosome-positive leukaemia to STI571: a prospective study. Lancet. 2002;359(9305):487–91.
19.
Shah NP, Sawyers CL. Mechanisms of resistance to STI571 in Philadelphia chromosome-associated leukemias. Oncogene. 2003;22(47):7389–95.PubMed
20.
Gambacorti-Passerini CB, Gunby RH, Piazza R, Galietta A, Rostagno R, Scapozza L. Molecular mechanisms of resistance to imatinib in Philadelphia-chromosome-positive leukaemias. Lancet Oncol. 2003;4(2):75–85.PubMed
21.
Branford S, Rudzki Z, Parkinson I, Grigg A, Taylor K, Seymour JF, et al. Real-time quantitative PCR analysis can be used as a primary screen to identify patients with CML treated with imatinib who have BCR-ABL kinase domain mutations. Blood. 2004;104(9):2926–32.PubMed
22.
Chu S, Xu H, Shah NP, Snyder DS, Forman SJ, Sawyers CL, et al. Detection of BCR-ABL kinase mutations in CD34+ cells from chronic myelogenous leukemia patients in complete cytogenetic remission on imatinib mesylate treatment. Blood. 2005;105(5):2093–8.PubMed
23.
Thomas J, Wang L, Clark RE, Pirmohamed M. Active transport of imatinib into and out of cells: implications for drug resistance. Blood. 2004;104(12):3739–45.PubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree