Implied risk of malignancy according to TBSRTC (%) [1]
Estimated risk based on reported studies (%) [5]
Atypia of unknown significance/follicular lesion of unknown significance (AUS/FLUS)
5–15%
6–48%
Follicular neoplasm/suspicious for follicular neoplasm (FN/SFN)
15–30%
14–34%
Suspicious for malignant cells (SMC)
60–75%
53–87%
The majority of these indeterminate nodules will undergo a repeat FNA and/or diagnostic lobectomy due to inability to rule out carcinoma from a FNA sample. Operations for indeterminate nodules that are benign may in retrospect be unnecessary. Indeterminate nodules that are benign may occur in up to 70–75% of patients undergoing diagnostic lobectomies [2]. As a result, researchers have been looking for methods to improve molecular testing to improve indeterminate thyroid nodule management.
Afirma Gene Expression Classifier (GEC)
The Afirma GEC is considered a thyroid cancer “rule out” test developed by Veracyte, Inc. (South San Francisco, CA). It was created by screening 240,000 genes and exon transcripts in 178 benign thyroid samples. After examining these samples, the 142 gene cDNA Affymetrix cassette was derived using a linear support vector machine model utilizing a recursive learning process from 167 mRNA expression patterns found in nodules with known benign histology [6, 7].
Test Characteristics
To perform the GEC test, a clinician obtains three designated FNA passes. One is designated for routine FNA cytomorphology, while two are stored in a RNA preservative for the multidimensional proprietary algorithm. The test has the ability to tolerate variations in RNA amount (4–25 ng) [8, 9]. The FNA sample first undergoes cytology testing at the centralized cytopathology lab, Thyroid Cytopathology Partners (Austin, TX). Samples classified as benign, malignant, or suspicious for malignancy do not undergo further testing, and the results are returned to the ordering physician. Indeterminate samples, according to TBSRTC described above (AUS/FLUS, FN/SFN), proceed to GEC testing [8].
During the first step of GEC testing, the sample is tested against a panel of 25 genes on six cassettes. This testing identifies thyroid nodules with expression profiles consistent with subtypes of follicular thyroid carcinoma (FTC) and non-follicular cell-derived thyroid tumors such as medullary thyroid carcinoma (MTC), oncocytic follicular (Hürthle cell) lesions, parathyroid tissue, as well as breast, renal cell, and melanoma metastasis. Afirma MTC evaluates expression of calcitonin-related polypeptide alpha (CALCA), carcinoembryonic antigen-related cell adhesion molecule 5 (CEACAM5), secretogranin III (SCG3), sodium channel voltage-gated type IX alpha subunit (SCN9A), and synaptotagmin IV (SYT4), which are all genes specific to MTC. If the expression of these genes is positive, GEC reports a positive “Afirma MTC” test in this first round of GEC reporting. If expression panels in one of the other five screening cassettes in the first round of GEC testing are positive, the nodule is reported as “suspicious” and does not undergo further testing. All other indeterminate samples proceed to the second round of GEC testing, which uses a support vector machine to classify the samples as benign or suspicious. The thyroid FNA sample is classified as “suspicious” if it has >50% risk of malignancy and “benign” if the negative predictive value is greater than 95% for AUS/FLUS or greater than 94% for FN. SMC nodules have a high pretest probability for malignancy, which results in a suboptimal NPV, and are not included in GEC testing [8–10].
Analytical validity of the Afirma GEC was studied by Walsh et al. and was found to meet the Evaluation of Genomic Applications in Practice and Prevention (EGAPP) level 1 analytic validity criteria [11]. The nucleic acids extracted were found to be stable, and the testing proved reproducible findings. To determine how much sample RNA would be required, samples were mixed with blood nucleic acid. GEC proved to hold up to dilution by maintaining the correct classification for up to 83% blood RNA. Limitations of GEC testing include the need for multiple needle passes [11].
Clinical Validity
Sensitivity, the probability of a positive test among persons with the disease, and specificity, the probability of a negative test among those disease-free, are both inherent qualities of all diagnostic tests and assumed to be independent of disease prevalence. However, the positive predictive value (PPV), the probability a patient who tested positive has the disease, and negative predictive value (NPV), the probability a patient who tested negative is disease-free, are dependent on the sensitivity, specificity, and disease prevalence in the patient population. For example, when the cancer prevalence is 80%, the PPV and NPV for the Afirma GEC test are 88.2% and 56.5%, respectively; however, when the prevalence decreases to 20%, the PPV decreases to only 31.9%, and the NPV increases 95.4% (Fig. 5.1). As the prevalence of malignant thyroid nodules may be significantly variable between populations and institutions studied, the utility of diagnostic tests must be carefully considered in the context of the population that is being applied [12].
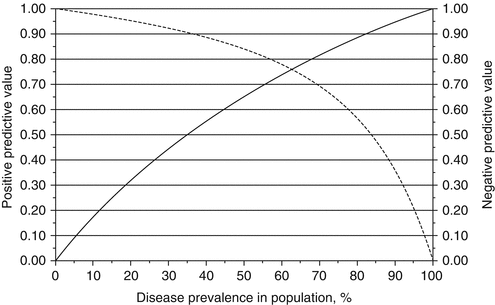

Fig. 5.1
The positive predictive value (solid line) and negative predictive value (dashed line) of the Afirma GEC test across disease prevalence, assuming a sensitivity and specificity of 90% and 52%, respectively [1]
In the first published study on Afirma GEC by the test creators, Chudova et al. described GEC AUS/FLUS and FN/Hürthle cell neoplasm (HCN) nodules to have a 5–6% posttest probability of malignancy for a “benign” result and 37–38% for a “suspicious” result [10]. These findings are more often described as a negative predictive value (NPV) of 94–95% and a positive predictive value (PPV) of 37–38% [10]. The 5–6% posttest probability risk of a GEC benign with cytological AUS/FLUS or FN/HCN is a risk deemed acceptable by the National Comprehensive Cancer Network to consider “observation in lieu of surgery” [13].
Afirma GEC was first clinically validated by Alexander et al. in a prospective, multicenter study examining 210 indeterminate nodules (AUS/FLUS and FN/SFN) [14]. In histopathology, 51 of the 210 thyroid samples were classified as malignant resulting in an overall 24% incidence of malignancy. The GEC evaluation correctly diagnosed 46 of the 51 malignant samples. The majority of false negatives were attributed to be suboptimal samples. Classification of the false negatives were as follows: three PTCs (size range, 0.6–1.2 cm), one follicular variant PTCs (size range, 1–3 cm), and one 3.5 cm Hürthle cell carcinoma [14].
For AUS/FLUS nodules, Alexander et al. showed GEC had a sensitivity of 90% (53% specificity) and NPV of 95% (38% PPV) in a population with a malignancy prevalence of 24%. Similar findings were seen in FN/SFN lesions, with a sensitivity of 90% (49% specificity) and NPV of 94% (37% PPV), with a malignancy prevalence of 25%. However in SMC nodules, there was a higher prevalence of malignancy (62%), which reduced the NPV to 85%. AS, the lower NPV in these nodules makes the findings unreliable, and GEC should not be used for indeterminate SMC lesions [14].
Clinical validity has been further studied in several subsequent studies. The study performed by Harrell et al. was limited by its small sample size of 36 suspicious GECs from Nova Southeastern University [15]. However, important results to note from this study were a disproportionate distribution of oncocytic lesions in the GEC suspicious category. Further evaluation proved that most of the oncocytic lesions were benign on the final pathologic evaluation. This study had a 51% malignancy prevalence and a NPV of 80%. This was the first study to highlight the significant decrease in NPV and thus limited utility when the GEC is used in a population with greater than 25% malignancy prevalence [15].
Marti et al. studied Afirma GEC at two tertiary care centers, Memorial Sloan Kettering (MSK) Cancer Center, a tertiary cancer referral center with a high prevalence of thyroid malignancy (55%), and the Mount Sinai Beth Israel, which has a low prevalence (10%). Since samples from MSK had a higher probability of malignancy, it resulted in a lower NPV (86–92%) and the inability of the GEC to sufficiently rule out cancer. In fact, to obtain a NPV >94% for the Afirma GEC, Marti et al. found that the pretest cancer risk (i.e., cancer prevalence) needs to be between 15% and 21% [12, 14]. This study reinforced that the NPV and PPV of a test are not uniformly applicable and vary widely across patient populations with different disease prevalence (Table 5.2).
Table 5.2
Afirma GEC studies and result for indeterminate nodules (AUS/FLUS and FN/SFN)
Study | Chudova 2012 [10] | Alexander 2012 [14] | Alexander 2014 [16] | Harrell 2014 [15] | McIver 2014 [9] | Lastra 2014 [17] | Marti [12] MSK | Marti [12] Mount Sinai Beth Israel |
N | 24 | 210 | 309 | 56 | 36 | 132 | 94 | 71 |
Prevalence (%) | 29 | 24 | 39 | 51 | 17 | 44 | 55 | 12 |
Sensitivity (%) | 100 | 90 | 98 | 94 | 83 | 100 | 100 | 100 |
Specificity (%) | 76 | 52 | 12 | 24 | 10 | 7 | 10 | 22 |
PPV (%) | 63 | 37 | 42 | 57 | 16 | 46 | 57 | 14 |
NPV (%) | 100 | 94 | 90 | 80 | 75 | 100 | 86–92 | 95–98 |
As highlighted by McIver et al. and Lastra et al., the majority of subsequent GEC studies have lacked surgical pathology correlation for a preponderance of benign GEC nodules [9, 17]. This means that due to the inability to identify true positives and false negatives, the sensitivity, specificity, PPV, and NPV could not be calculated [9, 17]. For example, while McIver et al. also found a significantly lower PPV of 16% with 80% of suspicious nodules found to be benign after surgery, it is unclear if this is due to a lower pretest probability, diminished sensitivity, and/or diminished specificity.
Clinical Applicability
Indications for the biopsy of thyroid nodules should continue to be determined according to the 2015 American Thyroid Association (ATA) guidelines [5]. GEC testing should be used when results of the test will influence management decisions, especially for nodules with indeterminate Bethesda criteria (AUS/FLUS and FN/SFN) in populations where the malignancy prevalence is ≤25%. For example, GEC testing would be especially useful for FN/SFN lesions when deciding between surgery and watchful waiting.
For AUS/FLUS and FN/SFN nodules that are GEC suspicious (Fig. 5.2), the 2015 ATA guidelines recommend at least a diagnostic thyroid lobectomy. If the nodule is GEC benign, active surveillance is advised, but diagnostic lobectomy may be appropriate for selected patients. Routine GEC testing is not recommended for SMC nodules [5]. Due to a lack of long-term follow-up on benign GEC results, both the ATA and the American Association of Clinical Endocrinologists continue to recommend long-term clinical and ultrasound follow-up [18].
Patients who should not undergo the Afirma GEC include those who have thyroid nodules that are greater than 4–5 cm due to increased sampling error and patients less than 21 years of age due to lack of clinical validation studies. For patients which, regardless of GEC results, surgery is indicated, GEC testing should not be performed. For example, patients with local or compressive symptoms, strong clinical or ultrasound findings consistent with malignancy, or in patients who desire surgery regardless of further testing [19].
Studies have reported mixed results whether GEC utilization has decreased the number of surgeries performed. Singer et al., Alexander et al., and Duick et al. showed significant decreases in surgery after GEC testing. Duick et al., a study funded by Veracyte, showed that the use of GEC testing decreased thyroidectomies (hemithyroidectomy and total thyroidectomies) from 74% for cytologically indeterminate nodules to 7.6% [20]. However, the lower rate of GEC benign nodules found in McIver et al. challenges Afirma GEC’s healthcare cost reduction by reducing one in four surgeries (16 of 48 nodules were benign) compared to the previously reported one in two surgeries seen in Alexander et al. [9, 14, 20]. Marti et al. also showed no significant difference in number of surgeries due to the high thyroid carcinoma prevalence at MSK and resulting low NPV [12, 16, 20, 21]. Given that healthcare costs have the opportunity to be reduced by $2600 per patient tested, using the 2010 Medicare estimates for thyroidectomy with complications ($12,000) and the predicted Medicare reimbursement for Afirma GEC ($3200), further exploration into the utility of Afirma GEC is needed [18, 20].
Future Direction
Afirma BRAF has been developed to supplement the Afirma GEC. BRAF mutations have nearly 100% specificity for thyroid malignancy and are present in approximately 45% of thyroid carcinomas. In addition, BRAF mutations have been associated with more aggressive behavior [22–24]. Thus, BRAF mutation testing has the potential to improve the diagnostic and clinical decision-making value of GEC [25].
Gene Mutation Panels
Genetic mutations are associated with 70–80% of malignant thyroid cancers. The 7-gene mutation panel evaluates thyroid FNA samples for BRAF, NRAS, HRAS, and KRAS mutations as well as translocations of the RET/PTC1, RET/PTC3, and PAX8/PPAR gamma genes [26]. The presence of BRAF mutation has a PPV of 99.3% for PTC, and the presence of RAS mutations has a 74–87% PPV for thyroid carcinoma [13, 26–28].
Test Characteristics
Currently there are multiple commercially available 7-gene mutation panels. Interpace Diagnostics’ ThyGenX (Parsippany, NJ), previously known as the miRInform test, requires one FNA sample with a minimum of 50 ng of cellular material to run multiplex polymerase cycling assembly (PCA) to detect mutations and rearrangements using sequence-specific probes. If ThyGenX is positive, the sample has the opportunity to undergo reflex ThyraMIR testing (Interpace Diagnostics, Parsippany, NJ) determined by provider preference in order to evaluate microRNA expression. ThyraMIR will be discussed in more detail later in the chapter [8]. Quest Diagnostics (Madison, NJ) performs a 7-gene mutation panel using DNA from formalin-fixed paraffin-embedded (FFPE) tissue blocks or four FNA samples. Real-time PCR and post-PCR melting curve analysis are used to detect mutations and rearrangements. PCR-based DNA Sanger sequencing is then performed to confirm genetic alterations. RET/PTC alterations are detected using fluorescence in situ hybridization (FISH) when FFPE samples are provided [29].
Clinical Validity
The original study testing the thyroid cancer gene mutation panel was performed by Nikiforov et al. This study used DNA from samples to test for BRAF V600E and K601E; point mutations in NRAS codon 61, HRAS codon 61, and KRAS codons 12 and 13; and RNA from samples to test for RET/PTC1, RET/PTC3, and PAX8/PPAR rearrangements using real-time PCR. For indeterminate FNA samples, the 7-gene panel showed a specificity of 100% and a PPV of 100% in 52 samples (prevalence 40%). Nodules with indeterminate cytology and lacking mutations had a 16% risk of malignancy when confirmed with surgical histology. Based on these findings, the researchers concluded there would be a 30% reduction in second surgeries for completion total thyroidectomies and no inappropriate total thyroidectomies would be performed. However, a major limitation to this study was the lack of blinding of the cytopathologist to the molecular mutation results [26].
Subsequently Nikiforov et al. evaluated the 7-gene panel with a larger sample size. Similar to the initial study, there was still no blinding of the cytopathologist. Lack of blinding promotes biased results if there is a tendency to look more closely or call borderline cases of cancer if there was evidence of mutation(s) [13]. All false positives (eight) in this study were attributed to RAS mutations in follicular adenomas without definitive features of malignancy based on histology. In an unrelated validation study performed by Cantara et al. in 2010 with 41 thyroid samples, the presence of any mutation resulted in a 91.1% PPV, and the false-positive results were follicular adenomas [30]. Mutations found in follicular adenomas were all attributed to RAS mutations and made up of 26% of all RAS mutations identified [18]. Some have hypothesized that RAS mutation-positive follicular adenomas may be precursor lesions to invasive lesions given the role of RAS in promoting malignant transformation and differentiation [13, 31, 32].
Studies performed by Eszlinger et al. showed that air-dried FNA samples were comparable to fresh FNA samples that had been previously studied for use in the 7-gene mutation panels. These studies were performed to evaluate the 7-gene mutation panel in a more routine daily lab practice setting. The initial retrospective study performed by Eszlinger et al. in 2014 showed a specificity of 86% and PPV of 19% with a 16% prevalence, which was significantly worse compared to prior reported gene mutation panel studies. In the follow-up prospective study performed by Eszlinger et al., the specificity improved to 92% and PPV to 71% (prevalence 28%) (Table 5.3).
Table 5.3
7-gene mutation panel studies and result for indeterminate nodules
Study | Nikiforov 2009 [26] | Cantara 2010 [30] | Nikiforov 2011 [13] | Beaudenon-Huibregtse 2014 [36] | Eszlinger 2014 [33] | Eszlinger 2015 [34] |
Design | Prospective, two centers | Prospective, single center | Prospective, single center | Prospective, multicenter | Retrospective, single center | Prospective, single center |
n | 52 | 41 | 513 | 55 | 141 | 163
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|