Fig. 1.1
Picture of a molecular breast imaging system. Courtesy of Jason Koshnitsky, Gamma Medica, Inc., Salem, NH
Indications
The indications for performance of a BSGI study include high-risk surveillance, alternative to MRI, palpable breast masses with a negative mammogram and ultrasound, a newly diagnosed breast cancer with occult foci, and in women with breast implants or following direct silicone injection to resolve a difficult question.
Initially studies were performed on a conventional gamma camera and hence there were issues related to optimal positioning of the breast and poor resolution; this technique did not detect small cancers less than 1 cm. The sensitivity was less than 50 %, making it a less attractive alternative [6, 9]. Over the last 20 years advancements in gamma ray detector technology (for example, the use of the semiconductor cadmium zinc telluride) and the use of dedicated dual head breast scanners have improved both energy and spatial resolution. This has enabled detection of tumors as small as 1–3 mm. Also, production of images that are oriented similar to standard mammograms has made it easier for radiologists to interpret these studies. These improvements have also resulted in decreased radiopharmaceuticals doses, making the test more acceptable [7, 16].
Technique
MBI uses the radiotracer technetium (99mTc) sestamibi in doses of about 20 mCi (740 mBq) injected intravenously (IV) in one of the antecubital veins. Imaging starts immediately and continues up to the desired number of counts per image, approximately 100,000. Images are obtained with breast oriented in a manner similar to the standard mammogram; craniocaudal (CC) and mediolateral (MLO) images of both breasts. The image acquisition time is about 10 min for each view, to a total acquisition time of 40 min for a complete study [9, 17]. The breast compression is less than that in a mammogram (a pressure of 15 lbs/square in. as opposed to 45 lbs/square in. on a conventional mammogram).
Advantages
In addition to its utility as an adjunct diagnostic tool, MBI is an attractive imaging test from the cost point of view because of the wide availability of the radiopharmaceutical and compact size of the imaging equipment. It is a useful problem-solving tool particularly in patients unsuited for MRI, because of metallic implants, renal dysfunction, or claustrophobia. BSGI has high sensitivity, specificity, and positive predictive value (PPV) compared to standard mammograms and ultrasound evaluation [7–9, 16–18]. Figure 1.2 shows an example. Weigert et al. [8] in a multicenter study determining the impact of molecular imaging concluded that statistically BSGI was more accurate (better sensitivity, specificity and PPV) than ultrasound, when findings were discordant from a standard mammogram. Lately, BSGI guided biopsy systems [6, 8] have become available which allows confirmation of pathology results and better correlation with imaging findings.
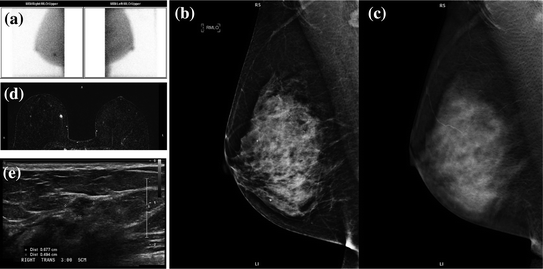

Fig. 1.2
An asymptomatic postmenopausal woman with a prior negative mammogram participated in a research study evaluating the effect of caffeine on Tc99 m sestamibi uptake. Her molecular breast imaging (MBI) exam (bilateral MLO) was positive (a). There was a focal area of moderate intensity radiotracer uptake in the lower inner right breast at middle depth measuring 1 × 0.7 × 0.8 cm. Subsequent digital mammogram (b), and digital breast tomosynthesis (c) showed no correlate to the MBI finding. Breast MRI (d) depicted a 0.6 × 0.6 × 0.9 cm enhancing round mass with slight irregular margins corresponding to the abnormality identified by MBI (a). Second look ultrasound (e) followed by ultrasound guided biopsy indicated a 6 mm invasive ductal carcinoma. Courtesy of Michael K. O’Connor, Ph.D., Mayo Clinic, Rochester, MN
Limitations
Poor visualization of chest wall and axilla are some of the drawbacks, which can be overcome with additional views. The inability to obtain all of the breast tissue in the field of view (FOV) to a large extent has been alleviated by offering nuclear medicine technologists training in breast positioning by mammographers [9]. In view of potential radiation risks the dose of the injected radiopharmaceutical has been steadily decreasing. Initial trials on BSGI used doses of 30 mCi (1110 mBq), currently this has dropped to 20 mCi and some centers use only 10–15 mCi of 99mTc [7]. Trials at Mayo Clinic in Rochester, MN are experimenting with a dose as low as 4 mCi and by enhancing image quality with digital post-processing. At this dose the radiation dose to the breast is comparable to a standard two-view mammogram. The results from this study will determine if MBI has a role in screening, particularly for the intermediate risk category, where the benefit of MRI is not clearly defined. While one of the earlier limitations of BSGI was the poor sensitivity of molecular imaging in identifying sub-cm tumors, with recent advances, Hruska et al. [16] demonstrated sensitivities up to 80 % for tumors less than 1 cm.
Positron Emission Mammography (PEM)
Positron emission tomography (PET) imaging is a useful diagnostic test in many malignancies, but has not been accepted as standard of care in breast cancers [19]. Dedicated PEM scanners for breast have been developed, providing higher resolution than whole body PET scanners [20]. In PEM and PET imaging, the radiotracer fluoro-deoxyglucose (18-FDG) is used, providing a physiological measure of increased metabolic activity. While a promising tool it also demonstrates ‘hot spots’ at inflammatory and infective sites resulting in false positives.
Limitations
In order for the test to be sensitive, it is essential that there is good regulation of glucose levels and the blood glucose levels must be below 120 mg/dl. In order to achieve sufficient gamma counts in the image it is necessary for the patient to wait at least 2 h after administration of the radiopharmaceutical before imaging the breast. Also, it is important for the patient to remain quiet and warm to prevent ‘hot spots’ from unusual muscular activity. One of the limitations of the earlier whole body PET scanners was its inability to pick up sub-cm cancers. This has been addressed to a large extent by the development of new, dedicated breast scanners. Also, low grade tumors and some invasive lobular cancers and ductal carcinoma in situ do not show avid uptake of the radiotracer [19]. Radiation concern continues to be a major limitation [10]. PEM suffers from some of the same limitations associated with positioning as noted in BSGI. Not only does the imaging take 40 min (10 min for each view), but the patient needs to wait about 2 h post tracer injection before the images can be obtained. Like BSGI, the sensitivity in PEM imaging showed a declining trend with smaller sized tumors. In addition, PEM equipment is more expensive than BSGI and requires access to the 18-FDG radiotracer. Hence, PET and PEM are available only in a limited number of clinical practices in the United States.
Advantages
The availability of more recent prototypes of dedicated PEM scanners with its ability to perform imaging guided biopsies has made it an attractive additional imaging tool [19, 21]. PEM sensitivity matched MRI for single lesions and the sensitivity for unsuspected lesion was around 85 % [22, 23]. PEM had higher specificity for unsuspected lesion compared to MRI. PEM imaging is useful in identifying the extent of the tumor and staging, evaluating response to treatment, identifying sites of distant metastases, and distinguishing a scar from recurrence [19, 20, 22, 23].
Research studies over the past 10–15 years have established the role and value of MBI and PEM in breast imaging. While the newer breast molecular imaging modalities have shown promise, they are still only useful as supplemental imaging tools that can increase the radiologists’ confidence in detecting breast cancer and cannot supplant established modalities such as screening mammogram, ultrasound, and MR imaging. Additional regulatory approvals are needed for the clinical site to handle radioactivity.
Radiation Risks
Since molecular imaging involves substantial radiation dose to part of the body other than the breast, there is concern about risk of cancer for radiosensitive organs; in the urinary bladder with PEM studies and in the colon with BSGI studies. Hendrick [10] has estimated the lifetime attributable risk (LAR) of a fatal cancer in BSGI studies at standard recommended doses of 20–30 mCi (740–1100 MBq) to be 20–30 times that of a digital mammogram in woman aged 40 years, and 23 times higher with a single PEM study at standard 10 mCi (370 MBq) dose of 18-FDG.
It is also relevant to add that even though considerable advancements have been made in radiotracer-based molecular imaging of the breast, currently it is not a screening tool. Its primary role may be as an adjunct to standard mammography and ultrasound, particularly in women with dense breasts and in the intermediate risk category. In the high-risk women, MRI with its proven track record as the modality with the highest sensitivity for detection is the established modality. Both BSGI and PEM/PET have the advantage of identifying physiological changes that distinguish a cancerous lesion from benign tissue and also identify additional foci in the ipsilateral and contralateral breast. They are also helpful imaging options to monitor response to chemotherapy drugs. Their sensitivity is however known to decrease with smaller sized tumors. A higher incidence of false positive tracer uptake has been noted in fibrocystic lesions, growing fibroadenomas, and fat necrosis. PEM has shown to be useful in distinguishing a scar from recurrence where conventional imaging findings are equivocal.
Optical Imaging with Near Infrared Spectroscopy
Transillumination of the breast using light, referred to as diaphanography, was proposed in 1920s. Variants of this approach were investigated till the early 1990s. However, the approach was not recommended for breast cancer screening [24]. Better understanding of the contrast mechanisms, characterization of absorption and scattering properties of breast tissues at various wavelengths, and techniques for modeling optical photon transport through tissues have facilitated development of quantitative methods for diffuse optical spectroscopic imaging. Diffuse optical imaging using continuous wave, time domain, or frequency domain measurements at near-infrared (NIR) wavelengths can be used to provide noninvasive in vivo quantitation of attenuation and scattering properties of breast tissue. This can be used to determine total hemoglobin content, oxygen saturation (ratio of oxygenated hemoglobin to total hemoglobin), water, and lipid content. Extension of the approach to 3D imaging, similar to computed tomography (CT), has resulted in diffuse optical tomography (DOT) systems. Hand-held diffuse optical spectroscopy imaging systems have been developed and continue to be refined [25, 26]. Stand-alone DOT prototype systems for adjunctive use in diagnostic breast imaging have been developed by academic investigators [27, 28] and by commercial entities (SoftScan®, Advanced Research Technologies, Inc., Montreal, Canada; CTLM®, Imaging Diagnostic Systems, Inc., Fort Lauderdale, USA).
In a study of 90 subjects, DOT showed that the ratio of total hemoglobin in the abnormality to that in the normal contralateral breast was statistically different for malignant tumors [29]. However, the study noted that there may be a resolution threshold of approximately 6 mm. Development of multimodality systems combining NIRS with X-ray imaging has been reported [30–32]. In a study of 189 breasts from 125 subjects including 51 breasts with lesions, a statistically significant increase was observed for total hemoglobin in malignant tumors larger than 6 mm compared to fibroglandular tissue [33]. A hand-held probe combining ultrasound with NIR imaging has been developed, and in a study of 65 subjects with 81 lesions significantly higher concentration of total hemoglobin was observed in malignancies than benign lesions [34].
Development of NIR systems integrated with MRI [35, 36] that incorporates image information from MRI during NIRS reconstruction as well as clinical evaluation of such multimodality systems have shown that malignant tumor exhibit higher concentration of total hemoglobin and lower oxygen saturation. The use of exogenous contrast agents such as indocyanine green as well as tumor-targeted contrast agents that are under development can preferentially enhance lesions and could improve differential diagnosis. Monitoring of neoadjuvant chemotherapy response with a hand-held diffuse optical spectroscopic imaging system in a limited dataset showed that significant changes in oxygenated hemoglobin could be observed approximately 90 days after initiation of therapy [37]. In order to reduce re-excision rates following breast conserving surgery (BCS), NIR-based optical imaging systems to assess tumor margins are being investigated [38, 39]. In summary, the past two decades have witnessed substantial improvement in optical imaging techniques using NIR spectroscopy, and its transition to routine use for some clinical applications is highly probable in the near future.
Optical Imaging with Confocal Microscopy
In the management of early breast cancer, BCS is the standard surgical procedure, where excision of the least volume of breast tissue free of tumor cells at the margins is the goal. However, the current surgical literature [40] estimates positive margins at surgery in the range of 20–70 %. This necessitates revision excision. Currently, the quickest means to evaluate tumor margins is the ‘traditional frozen section’. This process is however laborious, time-consuming, does not include the entire tumor surface, and is limited by freezing artifacts of the surgical margins. Cauterization surgery also limits evaluation. Currently, there are many experimental, real-time, imaging options that are being evaluated. There is a need for such techniques to be cost-effective, reproducible, and dependable.
We performed an experimental trial [41–43] of excised lumpectomy specimens at the University of Massachusetts Medical School in collaboration with the Medical Physics department at University of Massachusetts, Lowell, MA. The investigators evaluated lumpectomy specimens from breast cancer patients with polarization techniques after staining the specimen with dilute methylene blue. Wide-field polarization for quick macroscopic survey and small FOV confocal microscopy for small FOV with high resolution was performed, images analyzed, and later correlated with findings at Hematoxylin and Eosin (H&E) stained pathology slides evaluated by a trained breast pathologist (Fig. 1.3). The difference in the reflectance and fluorescence polarization values for benign and cancerous tissue was exploited. In these studies, Patel et al. [41–43] observed good correlation between fluorescence polarization values and findings on H&E stained sections of benign and malignant breast tissue on histopathology. The reflectance polarization values did not correlate as well. While the researchers concede to slight misregistration between confocal microscopy images and H&E stained specimens, the ease of use has good future potential to evaluate ex vivo specimens. The instrument could also be used in vivo on patients on the operating table to discern any residual malignant tissue that merits excision. A clinical trial is underway to evaluate the utility of this imaging technique for intraoperative evaluation of margins during BCS.
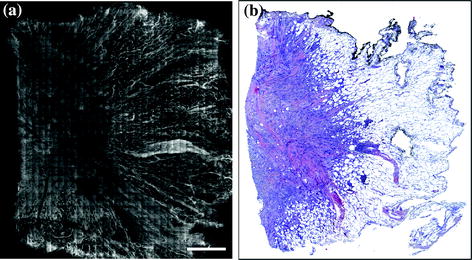

Fig. 1.3
Wide-field fluorescence polarization image (a) of a tissue section from a breast lumpectomy specimen with corresponding histopathology at scanning magnification (b) showing good demarcation between the benign (right half) and malignant (left half) breast tissue. Fluorescence polarization image courtesy Anna Yaroslavsky, Ph.D., University of Massachusetts, Lowell, MA
Terahertz Imaging
Accurate assessment of surgical margins of the excised breast specimen is very important in BCS in order to minimize the likelihood of re-excision. This is particularly relevant in the surgical treatment of invasive breast cancer followed by whole breast radiation therapy [44]. The reference standard for the determination of the tumor margins is by sectioning and imaging the excised specimen by conventional pathology procedures. However, more expedient techniques have been investigated over the years that allow prompt margin assessment at the intraoperative stage, thus affording the opportunity for the surgeon to excise additional tissue if needed. Breast specimen radiography has been used for many years for this purpose. This approach is used routinely in clinical practice but it has certain inherent limitations. Radiography generates planar images of a thick three-dimensional specimen; it provides good contrast for identification of surgical margins on the basis of changes in tissue composition and density, but it is not known to differentiate well between normal tissue and cancer especially when the malignancy does not exhibit prominent morphologic changes in tissue composition and density.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree