Fig. 20.1
Electron microscopy analysis. Mature adipocyte has thin cytoplasm and a nucleus characteristically squeezed by the large lipid vacuoles. The cytoplasmatic organelles are poorly represented. Scale bar = 4 μm
To obtain isolated mature adipocytes free of stromal-vascular elements, after collagenase digestion, the disrupted tissues were filtered through a 200 μm nylon sieve. The filtered cells should be washed four–five times and eventually re-filtered if it is necessary. Only the floating top layer was collected after each washing step (Zhang et al. 2000).
The confocal microscopy analysis of adipogenic cellular fraction isolated is a good and easy way to exclude any contamination coming from other cell types contained in the whole tissue. These cells are large (50–70 μm), unilocular, perilipin-immunoreactive with a peripheral flattened nucleus.
The ceiling culture is the method that uses the buoyant property of adipocytes, allowing them to adhere to the top inner surface of a culture flask which is completely filled with medium (Sugihara et al. 1987). In this way, mature adipocytes adhere to the ceiling surface, then the flask should be reinverted to allow normal observation and the subsequent manipulation of the culture.
The aim of this chapter is to collect and compare the literature data about both mature and dedifferentiated adipocytes to better underline their stem cells properties. In addition to well characterize adipocytes morphologically and structurally, this chapter reports on the pluripotent differentiation ability, immunoregolatory and hematopoietic supporting functions of these cells. These findings open new perspectives on adipose tissue plasticity and highlight the way for cellular therapy and regenerative medicine based on the administration of adipose tissue stem cells.
The Dedifferentiation Process of Mature Adipocytes
Mature adipocytes are generally considered terminally differentiated because they have lost their proliferative abilities, but recent data suggest that the process of cellular differentiation in terminally differentiated mammalian cells is not irreversible.
When maintained in culture, mature adipocytes undergo spontaneously a process of dedifferentiation (Matsumoto et al. 2008). Adipocytes lost their intracytoplasmatic lipid droplet, the nuclei became more centralized and the cells became elongated in shape, assuming a fibroblast-like morphology. At this step, the cells enter a proliferative log phase until cellular senescence (Fig. 20.2).
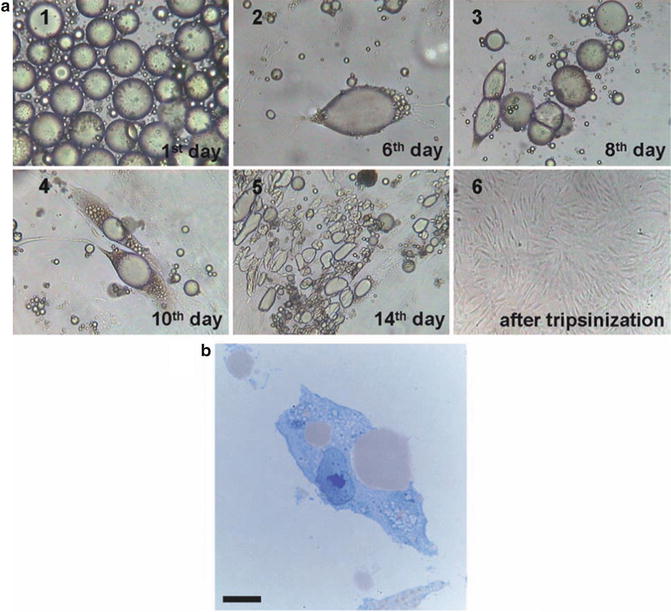

Fig. 20.2
Dedifferentiation process of mature adipocytes. During culturing, the cells attached to the upper surface of the flasks, followed by conversion to fibroblast-like dedifferentiated adipocytes, reached a morphology similar to BM-derived MSCs. (a) Morphological changes at different time points from mature adipocytes to dedifferentiated adipocytes. (b) Mature adipocytes lose their lipid droplets. Cells were stained by toluidine blue. Scale bar in B is equal to 4 μm, in A1 equal to 80 μm, in A2 equal to 40 μm, in A3 equal to 80 μm, in A4 equal to 80 μm, in A5 equal to 120 μm, and in A6 equal to 150 μm
At molecular level, data revealed significant changes in gene expression during the dedifferentiation process (Ono et al. 2011; Poloni et al. 2012). Adipocytes downregulated many genes that play a role in lipid and fatty acid metabolism, while concomitantly upregulated genes involved in cell proliferation, altered cell morphology, movement and migration of cells, and regulation of differentiation.
As murine adipocytes (De Matteis et al. 2009), recent data demonstrated that also human mature adipocytes expressed genes required for the cell reprogramming process, which include Oct4, Klf4, c-myc and Sox2 (Poloni et al. 2012, 2013). Moreover, these cells expressed transcripts for embryonic stem cell genes that are required for self-renewal and pluripotency. Thus, recent data underline the potential role of mature adipocytes as stem cells (Poloni et al. 2012; Cinti 2009). The reprogramming of genes in isolated as well as cultivated adipocytes is in line with the plastic properties of these cells.
Immunophenotype Characterization and Pluripotential Properties of Mature and Dedifferentiated Adipocytes
While dedifferentiated fat cells are well characterized (Matsumoto et al. 2008), there are only few studies that provided the cell-surface antigen profile of human mature adipocytes (Poloni et al. 2012). The stemness markers expressed by mature adipocytes at the molecular level are also present as surface antigens. These cells are positive stained by some stem cells markers, CD34 (hematopoietic progenitor cell antigen 1, HPCA 1), CD133 (prominin 1), CD90 (Thy-1), CD105 (endoglin), CD271 (NGFR) and CD117 (c-kit). During the dedifferentiation process, the typical mesenchymal stem cells markers are highly preserved at the molecular and antigenic levels, while CD34 and CD133 are lost as antigens.
After the dedifferentiation process, adipocytes are easy to isolate and cultivate, so there are many studies that described their immunophenotype. The cell-surface antigen profile of dedifferentiated cells was analyzed (Matsumoto et al. 2008) and this profile is consistent with previous findings for bone marrow MSCs (Pittenger et al. 1999) with uniformly positive expression of CD13 (aminopeptidase N), CD29 (integrin β1), CD44 (hyaluronate receptor), CD49b (integrin α4-subunit), CD90, CD105, CD271, CD73 (ecto-5′-nuclotidase) and HLA-A, -B, -C, but negative for CD11b (integrin αM), CD31 (platelet endothelial cell adhesion molecule), CD34, CD133, CD45 (leukocyte common antigen), CD106 (vascular cell adhesion molecule-1) and HLA-DR.
These findings suggest that the dedifferentiated adipocytes are an homogeneous population expressing the same markers as bone marrow-derived MSCs and adipose tissue-derived MSCs. Data showed that dedifferentiated adipocytes are a highly homogeneous population of cells respect to adipose-derived stem cells, that contained a variety of cell types: high number of smooth muscle cells (19%), endothelial cells (3%) and blood cells (13%) (Yoshimura et al. 2006). These observations are convincing because dedifferentiated adipocytes originate from a fraction of highly pure mature adipocytes, whereas adipose-derived stem cells are an heterogeneous population.
Electron microscopy (EM) analysis of dedifferentiated adipocytes is shown in Fig. 20.3a, b. The images show most organelles described during the early stages of developing stromal vascular fraction-derived MSCs in primary cultures, i.e., well-developed Golgi complexes, short strands of rough endoplasmic reticulum, small lipid droplets, small mitochondria, lysosomes and small granules of glycogen. Nuclei were fusiform with smooth edges. Thus, the EM features of dedifferentiated adipocytes were very similar to developing stromal vascular fraction-derived MSCs (Fig. 20.3c). The spontaneous dedifferentiation process represents the manifestation of morphological, molecular and functional changes of mature adipocytes, that might be interpreted as a return back to a noncommitted status of the cells.
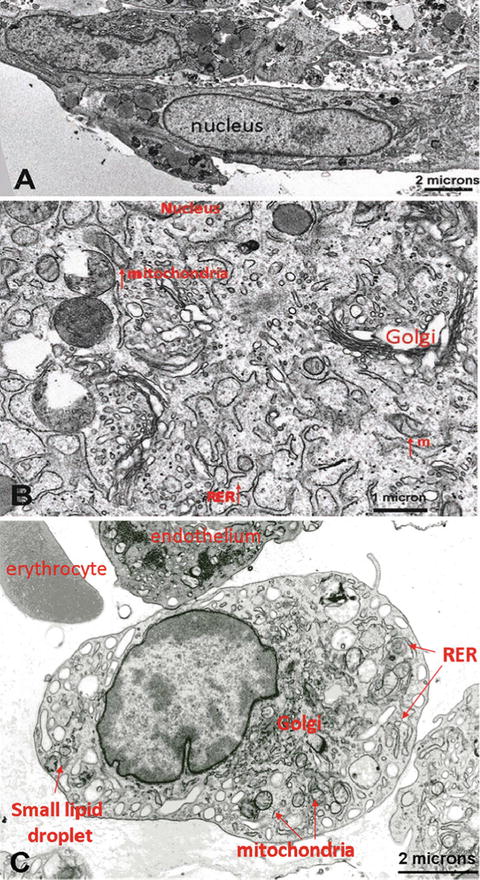

Fig. 20.3
Electron microscopy analysis. (a) Dedifferentiated adipocytes displayed structural organelle similarities (cytoplasm enlarged in b) to stromal vascular fraction-derived MSCs (c); RER rough endoplasmic reticulum, m mitochondria
The methylation status of cells is the most common epigenetic modification of genome in mammalian cells (Bibikova and Fan 2010). Data showed that there are significant difference between the methylation statuses of mature adipocytes and dedifferentiated adipocytes, while there are no difference between dedifferentiated adipocytes and bone marrow-derived MSCs (Poloni et al. 2012). These findings suggested that during the dedifferentiation process a gene reprogramming event takes place, which leads to changes in cellular epigenetic status. Dedifferentiated adipocytes achieve the DNA methylation status of bone marrow-derived MSCs by this process.
Recent advances in regenerative medicine have created a broad spectrum of stem cell research. Among them, tissue stem cell regulations are important issues to clarify the molecular mechanism of differentiation. Tissue engineering and cell therapy techniques have been developed to reconstitute different tissues. Attention has been focused on cells that might be useful in regenerative medicine (Barrilleaux et al. 2006). However, because stem cells only represent a minor population of the cells in the body, invasive procedures are frequently needed to obtain the amount of stem cells required for cell therapy. Therefore, adult stem cells sources are needed to be easily isolated and expanded with high purity.
Several studies have demonstrated that mesenchymal progenitor cells from various tissues have the potential to differentiate into other cells, suggesting that pre-committed and committed cells have plasticity in cell fate determination (Sudo et al. 2007).
Mature adipocytes are the most abundant cell type in adipose tissue and they can be easily isolated without painful procedures or donor site injury. Recent data demonstrated that mature adipocytes are able to transdifferentiate into another cell type, suggesting a great plasticity of these cells. This process implies an in vivo phenomenon of physiological and reversible reprogramming of genes in mature cells (Cinti 2009). During this process a differentiated cell turns phenotypically and functionally into a differentiated cell of another type without undergoing dedifferentiation. Some other include the additional step of dedifferentiation (Tosh and Slack 2002).
Data showed two important examples of physiological and reversible transdifferentiation. In conditions of chronic cold exposure the amount brown adipose tissue (BAT) in the organ could increase via white-to-brown transdifferentiation and, vice versa, BAT could turn into WAT in case of exposure to an obesogenic diet, to enable greater energy accumulation (Cinti 2011). Moreover, murine mature adipocytes undergo a reversible process of adipocytic/epithelial differentiation during pregnancy and lactation. During pregnancy, adipocytes seem to transform progressively into epithelial cells, forming the lobolo-alveolar part of the mammary gland responsible for milk production. At the end of lactation the lobolo-alveolar component disappears and a new adipocyte population restores the prepregnancy anatomy (Cinti 2011).
While little is known about the potential differentiation of mature adipocytes, more informations are available regarding the dedifferentiated population. Because adipose tissue is abundant and easily accessible tissue at most ages, dedifferentiated cells may be an attractive source of mesenchymal lineages for tissue engineering and other cell-based therapy (Matsumoto et al. 2008). The plastic properties of mature adipocytes are present also in dedifferentiated adipocytes.
In line with these observations, results showed that dedifferentiated adipocytes can be converted into fully differentiated cells, like adipocytes both in vitro and in vivo (Fernyhough et al. 2008), chondrocytes and skeletal myocytes in vitro (Kazama et al. 2008) under the appropriate culture conditions. In particular, dedifferentiated cells may be applicable to bone tissue engineering startegies and cell-based therapies, because they could convert into differentiated osteoblasts in vitro only by transient all-trans retinoic acid stimulation. Thus, dedifferentiated cells can undergo terminal osteoblast differentiation and osteoblast matrix formation, following subcutaneous injection into the peritoneal cavity of mice (Oki et al. 2008).
Moreover, dedifferentiated adipocytes also have the potential to rapidly acquire the endothelial phenotype in vitro and to promote neovascularization in ischemic tissue and vessel-like structure formation, suggesting that cells of endothelial and adipocyte phenotype may have a common precursor (Planat-Benard et al. 2004). These results also highlight the concept that adipose lineage cells represent a suitable new source for therapeutic angiogenesis in ischemic disease.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree