Fig. 9.1
CC chemokine receptor 4 (CCR4) and humanized anti-CCR4 monoclonal antibody, KW-0761. The CCR4 gene is located on chromosome 3p24. CCR4 is a seven-domain transmembrane G protein-coupled receptor, and TARC/CCL17 and MDC/CCL22 are ligands of CCR4. The anti-CCR4 monoclonal antibody recognizes the N-terminal portion of the CCR4 molecule
9.3 Phase I Study in Relapsed ATL
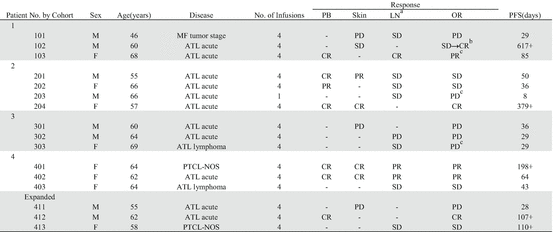
This phase I study assessed the safety, pharmacokinetics, and recommended phase II dose and efficacy of KW-0761 (mogamulizumab) in patients with relapsed CCR4-positive adult T-cell leukemia-lymphoma (ATL) or peripheral T-cell lymphoma (PTCL) [15]. In this study, 16 patients received KW-0761 once a week for 4 weeks by intravenous infusion (Table 9.1). Doses were escalated, starting at 0.01, 0.1, 0.5, and finally 1.0 mg/kg by a 3 + 3 design (Fig. 9.2). Fifteen patients completed the protocol treatment. Only one patient, at the 1.0 mg/kg dose, developed grade 3 dose-limiting toxicities, skin rash, and febrile neutropenia and grade 4 neutropenia. Other treatment-related grade 3–4 toxicities were lymphopenia (n = 10), neutropenia (n = 3), leukopenia (n = 2), herpes zoster (n = 1), and acute infusion reaction/cytokine release syndrome (n = 1). Neither the frequency nor severity of toxicities increased with dose escalation. The maximum tolerated dose was not reached. Therefore, the recommended phase II dose was determined to be 1.0 mg/kg. No patients had detectable levels of anti-KW-0761 antibody. The plasma maximum and trough, and the area under the curve of 0–7 days of KW-0761, tended to increase dose and frequency dependently. Five patients (31%; 95% CI, 11–59%) achieved objective responses: two complete (0.1; 1.0 mg/kg) and three partial (0.01; 2 at 1.0 mg/kg) responses (Table 9.2). KW-0761 was tolerated at all the dose levels tested, demonstrating potential efficacy against relapsed CCR4-positive ATL or PTCL. Subsequent phase II studies at the 1.0 mg/kg dose are thus warranted.
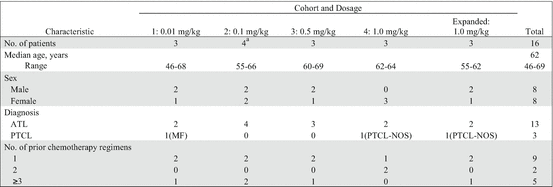
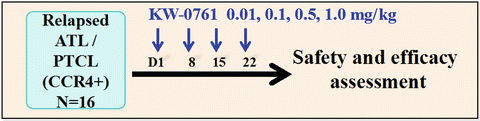
Table 9.1
Patient demographic and clinical characteristics by cohort in phase I study in relapsed ATL (Ref. [15])


Fig. 9.2
Study design of phase I study of mogamulizumab in relapsed ATL and PTCL. This was a multicenter dose-escalation study with three to six patients at each dose level to determine the maximum-tolerated dose (MTD) and estimate the recommended phase II dose. Cohorts of patients received KW-0761 at 0.01, 0.1, 0.5, and 1.0 mg/kg, weekly for 4 weeks by intravenous infusion. Premedications (antihistamine and antipyretic) were administered before each KW-0761 (mogamulizumab) treatment
9.4 Phase II Study in Relapsed ATL
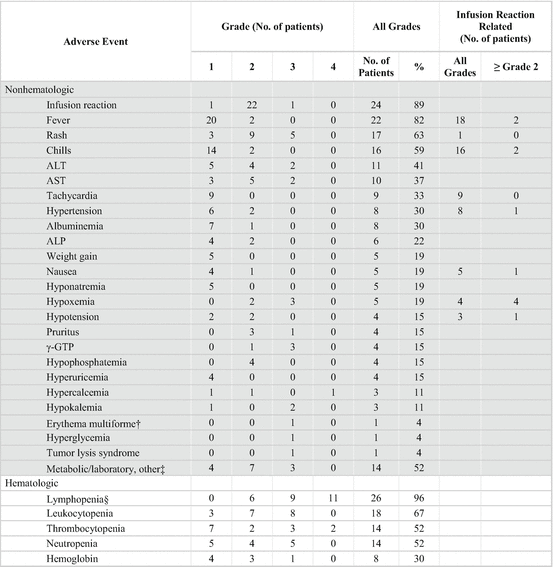
A multicenter phase II study of KW-0761 for patients with relapsed, aggressive CCR4-positive ATL was conducted to evaluate efficacy, pharmacokinetic profile, and safety [16]. The primary end point was overall response rate, and secondary end points included progression-free survival and overall survival from the first dose of KW-0761. Patients received intravenous infusions of KW-0761 once per week for 8 weeks at a dose of 1.0 mg/kg (Fig. 9.3). Of 28 patients enrolled onto the study, 27 received at least one infusion of KW-0761 (Table 9.3). Objective responses were noted in 13 of 26 evaluable patients, including eight complete responses, with an overall response rate of 50% (95% CI, 30–70%). Median progression-free survival and overall survival were 5.2 and 13.7 months, respectively (Fig. 9.4). The mean half-life period after the eighth infusion was 422 ± 147 h (± standard deviation). The most common adverse events were infusion reactions (89%) and skin rashes (63%), which were manageable and reversible in all cases (Table 9.4). KW-0761 demonstrated clinically meaningful antitumor activity in patients with relapsed ATL, with an acceptable toxicity profile. Further investigation of KW-0761 for treatment of ATL and other T-cell neoplasms is warranted.
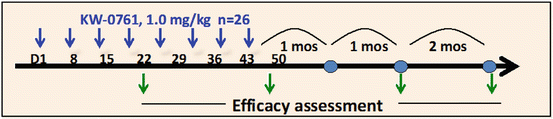
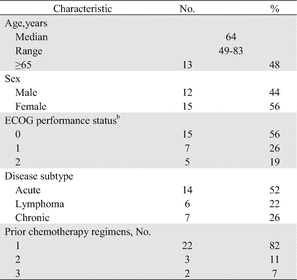
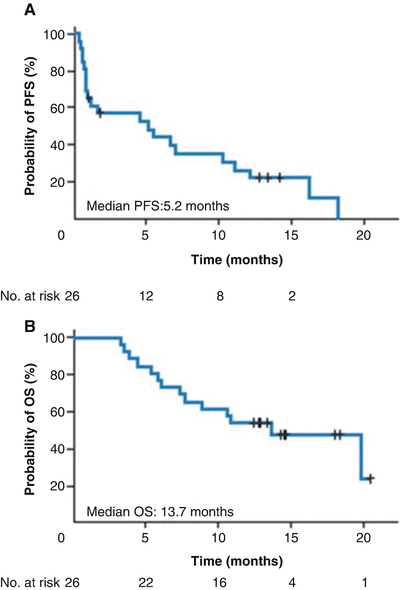

Fig. 9.3
Study design of phase II study. This study was a multicenter, single-arm, phase II trial. Objectives of the study were to evaluate the efficacy, pharmacokinetic profile, and safety of KW-0761 monotherapy. Patients received intravenous infusions of KW-0761 once per week for 8 weeks at a dose of 1.0 mg/kg
Table 9.3
Patient demographics and clinical characteristics in phase II study in relapsed ATL (n = 27)a (Ref. [16])


Fig. 9.4
Kaplan-Meier curves of estimated (a) progression-free survival (PFS; median, 5.2 months) and (b) overall survival (OS; median, 13.7 months) in phase II study in relapsed ATL (Ref. [16])
Base on these phase I and phase II studies, mogamulizumab was approved and launched on May 2012 in Japan.
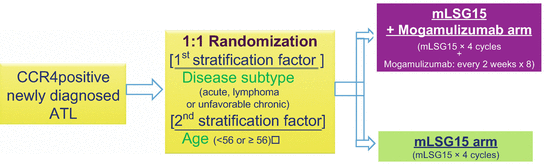
9.5 Randomized Phase II Study in Newly Diagnosed ATL
This multicenter, randomized, phase II study was conducted to examine whether the addition of mogamulizumab, a humanized anti-CC chemokine receptor 4 antibody, to mLSG15, a dose-intensified chemotherapy, further increases efficacy without compromising safety of patients with newly diagnosed aggressive adult T-cell leukemia-lymphoma (ATL) (Fig. 9.5). Patients were assigned 1:1 to receive mLSG15 plus mogamulizumab or mLSG15 alone. The primary end point was the complete response rate (%CR); secondary end points included the overall response rate (ORR) and safety. Between August 2010 and September 2011, 54 patients with newly diagnosed aggressive ATL were enrolled at 18 institutions. Of these 54 patients, 29 in the mLSG15-plus-mogamulizumab arm and 24 in the mLSG15 arm received treatment according to our study protocol (Table 9.5). The %CR and ORR in the mLSG15-plus-mogamulizumab arm (n = 29) were 52% [95% confidence interval (CI), 33–71%] and 86%, respectively; the corresponding values in the mLSG15 arm (n = 24) were 33% (95% CI, 16–55%) and 75%, respectively (Table 9.6). The median PFS in the mLSG15-plus-mogamulizumab and mLSG15 arms were 8·5 and 6·3 months, respectively (Fig. 9.6). The median OS was not reached in either arm (Fig. 9.6). Grade ≥3 treatment-emergent adverse events, including anemia, thrombocytopenia, lymphopenia, leukopenia, and decreased appetite, were observed more frequently (≥10% difference) in the mLSG15-plus-mogamulizumab arm. Several adverse events, including skin disorders, cytomegalovirus infection, pyrexia, hyperglycemia, and interstitial lung disease, were observed only in the mLSG15-plus-mogamulizumab arm. Although the combination strategy showed a potentially less favorable safety profile, a higher %CR was achieved, providing the basis for further investigation of this novel treatment for newly diagnosed aggressive ATL. Based on these data of this randomized phase II study, mogamulizumab was approved for the expansion of indication in newly diagnosed CCR4+ ATL on December 2014 in Japan.