Keywords
Herpes simplex virus, immunotherapy, oncolysis, advanced cancer, vaccine
Introduction
Recent advances in viral genome sequencing and elucidation of viral protein function combined with a better understanding of tumor genetics and tumor immunology have renewed enthusiasm for oncolytic virus therapy. Elements of tumor tropism determinants have been better characterized, resulting in improved tumor targeting while increasing lytic properties, which has led to more potent oncolytic vectors. The ability to delete pathogenic viral genes has added an element of increased safety for the clinical development of oncolytic viral therapy in the clinic. Furthermore, progress in understanding the genetic basis of individual tumor cells and new insights into the interaction of tumor cells with the host immune system have aided in designing optimal clinical trials and new therapeutic strategies. Herpes simplex viruses (HSV) are particularly well suited as oncolytic vectors and are now in advanced phase clinical trials for melanoma and being actively evaluated in a variety of other cancers.
In addition to the oncolytic activity of HSV vectors, activation of the host antitumor immune system is important for clearance of inaccessible visceral tumors. Immune system activation, via exposure to small amounts of antigen, is the basis of vaccination and provides protection against specific infectious disease pathogens and possible tumor-associated antigens. The application of this concept to cancer therapy—by manipulating tumor antigens to activate the immune system—has had mixed clinical results . Likely, this was secondary to an inability of the immune system to overcome the immunosuppressive milieu of the tumor . New-generation modified oncolytic HSV vectors can preferentially replicate in tumor cells, cause tumor cell death, and disrupt the immunosuppressive tumor microenvironment with release of tumor antigens. Released antigen can then be processed by local dendritic cells and presented to T cells, inducing systemic antitumor immunity. This has been most clearly demonstrated in preclinical and clinical studies of a modified oncolytic herpesvirus encoding human granulocyte–macrophage colony-stimulating factor (GM-CSF) in melanoma. This chapter reviews the basic biology and modifications of HSV with a focus on optimizing the oncolytic and therapeutic potential of the virus, and it discusses clinical results from cancer trials with an emphasis on melanoma.
Basic Virology of HSV
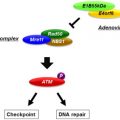
Herpes is derived from the Greek word Herpein , to creep, and this describes the creeping or spreading nature of the skin lesions often associated with herpes infections. The herpes family can be subdivided into three major subfamilies that include alpha-, beta-, and gamma-herpesviruses and some genera that are not otherwise classified. The herpesviruses include simplex and varicella species. These viruses are a major cause of human disease, which is generally manifest as skin lesions and rashes. Infection typically occurs in the mucosal surface and results in skin-related vesicular lesions. The viruses can enter peripheral neurons through a process called retrograde axoplasmic flow, in which the viruses can stay for many years and become reactivated. Lymphocytes and macrophages are also susceptible to herpesvirus infection and may serve as a latent reservoir for viral particles. The herpes simplex virus type-1 (HSV-1) is well-known as a minor human pathogen and is responsible for the familiar cold sore. The virus consists of three major structural components—a central core where viral DNA sits, a surrounding envelope composed of glycoproteins and host cell membrane fragments, and a capsid. There is an area known as the tegument between the capsid and the envelope that contains proteins that are released into infected cells following viral entry. The HSV-1 is a double-stranded DNA virus with a genome size of approximately 152-kb that contains approximately 90 essential (viral growth) and nonessential (virus–host interactions) genes. Viral replication occurs in the nucleus, but insertional mutagenesis does not occur. The large size of the HSV-1 genome, which includes expanses of non-encoding regions (~30 kb nonessential), and the lack of insertional mutagenesis make it an attractive vector for recombinant vaccine development . HSV can persist for years in humans and generally does not induce neutralizing antibodies, allowing for repeated dosing.
HSV causes lytic and latent infections, with viral replication generally occurring within 15 hr of exposure. In the lytic phase, the virus typically infects mucosal epithelial cells, with type 1 more common in the oral cavity and mouth and type 2 more common in the genitalia, although both types may infect any mucosal cells. Epithelial cell entry occurs through binding of viral surface glycoproteins (called gB, gC, and gD) to host cell entry receptors. To date, three entry receptors have been described: heparan sulfate, herpesvirus entry mediator (HVEM), and nectins. HVEM is a member of the tumor necrosis factor superfamily and is highly expressed on natural killer (NK) and naive CD8 + T cells. It can also be found at reduced levels on CD4 + T cells, dendritic cells, B cells, fibroblasts, and epithelial cells. Nectin-1 and nectin-2 are members of the immunoglobulin superfamily and are found on epithelia cells, neurons, and fibroblasts. Following infection, the mucosal epithelial cell is lysed, releasing progeny viral particles. Latent infection occurs when virions enter sensory neurons, in which replication is infrequent because these cells do not divide. During latent infection, latency-associated transcripts are expressed and encode proteins that promote neuron and viral survival and are needed for reactivation of the viral infection. Reactivation occurs during times of stress and exposure to heat, ultraviolet light, fever, hormonal changes, and nerve trauma. The viral particles travel in an anterograde manner back to the epithelial cells to form the characteristic herpetic lesions. Herpesviruses induce immune responses, and most adults do have evidence of antibody titers against HSV-1.
Evolution of Modified HSV Vectors for Oncolytic Therapy
JS1/ICP34.5-/ICP47-/GM-CSF (Talimogene Laherparepvec (TVEC))
Originally isolated from human cold sores, JS1, the backbone of the virus, is a virulent strain of HSV that has been found to have superior tumor cytotoxicity in vitro . In this engineered stain, the ICP34.5 gene, responsible for the neurotoxic side effects of HSV, has been deleted . ICP34.5 is also implicated in HSV replication when it forms a complex with low levels of proliferating cell nuclear antigen (PCNA), a protein important in DNA repair and replication . This function is critical to allow the virus to continue to replicate in the presence of interferon-mediated antiviral immune responses. In malignant cells, however, HSV-1 is able to replicate in the absence of ICP34.5 expression due to high levels of PCNA. This selective ability to replicate in malignant cells is advantageous for an HSV-based vaccine . To increase viral replication and tumor killing, ICP 47 was also deleted. This serves two functions. First, it allows for upregulation of US11, a protein important for viral replication at an earlier time point in tumor cells . Second, deletion of ICP47 enhances antigen presentation by HSV-infected cells, enhancing immunogenicity.
Although the previously mentioned modifications enhance local tumor lysis, heightened systemic antitumor responses are also important for long-term protection against recurrence. GM-CSF has multiple roles, including activation of macrophages, recruitment of peripheral blood monocytes, and activation of dendritic cells that present antigens, resulting in a T cell-mediated immune response . Several clinical trials using GM-CSF monotherapy in patients have had varying results. However, the largest of these studies, E4697 (phase III group study), indicated that there is a selective benefit for patients with metastatic melanoma in subset analysis. This has yet to be confirmed in a prospective randomized trial . Given its role as an immune stimulant, GM-CSF has been used as a vaccine adjuvant. Insertion of GM-CSF into the HSV-1 strain JS1/ICP34.5-/ICP47- has led to development of JS1/ICP34.5-/ICP47-/GM-CSF, now named talimogene laherparepvec (TVEC). A description of the relevant gene deletions is provided in Table 15.1 . This particular strain has shown significant tumor regression in the A20 lymphoma mouse model. In particular, JS1/ICP34.5-/ICP47-/GM-CSF demonstrated greater therapeutic activity against noninjected contralateral tumors than the parental JS1/ICP34.5-/ICP47- strain without GM-CSF included . TVEC is currently in advanced phase clinical trials for varying tumor types that will be described later.
| Strain | Gene | Modification | Effect |
|---|---|---|---|
| JS1/ICP34.5-/ICP47-/GM-CSF (talimogene laherparepvec (TVEC)) | ICP34.5 | Deletion | Prevents HSV infection in nonmalignant cells and enhances tumor cell killing |
| GM-CSF | Insertion | Produces local GM-CSF to recruit and activate immune cells (e.g., dendritic cells) in the tumor microenvironment | |
| ICP47 | Deletion | Improve immune response by promoting antigen presentation | |
| US11 | Insertion | Bypasses PKR to allow viral protein synthesis | |
| HSV17 + and G207 | ICP34.5 | Deletion | Prevents HSV infection in nonmalignant cells and enhances tumor cell killing |
| NV1020 | HSV1 strain F genome | Deletion | Preserves viral replication processes while reducing neurotoxicity |
| Thymidine kinase | Insertion | ||
| HF10 | UL56 | Deletion | Interferes with intracellular protein transport, reducing neurotoxicity |
| rRp450 | UL39 | Deletion | Selective replication in rapidly dividing cells |
| CYP2B1 | Insertion | Potentiates the antitumor effect of cyclophosphamide and dampens host response to the virus |
HSV17 + and G207
The HSV oncolytic vaccines have undergone several modifications in order to optimize clinical efficacy. Several strains with varying genetic alterations have entered human clinical trials for a number of malignancies. HSV17 + and G207 are serially passaged strains of HSV1 from nonhuman cells that were used in phase I studies of multiple tumor types (primarily glioma and melanoma) . ICP34.5, the gene responsible for the neurotoxic side effects of HSV, was removed in these early HSV generation vaccines. This deletion resulted in no adverse neurological events in all preclinical and clinical trials. Although not as oncolytic as JS1, it has demonstrated some tumor response in clinical trials, as described in the next section.
NV1020
Another HSV strain in phase I/II clinical trials, NV1020, is a highly attenuated derivative of wild-type HSV1 that is multimutated and replication competent. This virus is based on R7020, which contains two deletions of the HSV-1 strain F genome (internal repeat of the UL56 gene and a gene that encodes thymidine kinase). A functional thymidine kinase HSV-1 gene was inserted in the deleted internal repeat region. These modifications ensured a replication-competent but significantly less pathogenic virus that could be controlled by drugs such as acyclovir if an infectious outbreak occurred .
HF10
This strain of HSV-1 has two key deletions resulting in two incomplete copies of UL56 without a promoter. The UL56 protein has been described as a tail- anchored type II membrane protein found in the Golgi apparatus and endosomes, lending itself to involvement in anterograde axonal transport of viral envelope glycoproteins. The alterations in the genomic structure of HF10 lead to a lack of UL56 protein product and a significant reduction in the neurological side effects of HSV-1 without affecting viral replication .
rRp450
This oncolytic herpes simplex virus was engineered to have the gene (UL39) that encodes a large subunit of ribonucleotide reductase (ICP6) deleted. These alterations allow for selective replication in rapidly dividing cells. This gene was then replaced by the rat CYP2B1 gene, which encodes a cytochrome P450 enzyme that activates oxazophosphorines. Cyclophosphamide is an example of an oxazophosphorine, and in preclinical studies rRp450 potentiates the antitumor effect of cyclophosphamide as well as dampening the host response to the virus and allowing for enhanced viral replication. This is in addition to the virus’s own directly oncolytic effects on the tumor .
Clinical Results of HSV Oncolytic Virus Therapy for Cancer
Melanoma
The potential role of HSV-based vaccines in cancer therapy, particularly TVEC, is most notable in melanoma clinical trials, the earliest of which used the HSV1716 strain in a 5-patient metastatic melanoma phase I trial. Patients received up to three injections of 10 8 plaque-forming units (PFU) of vaccine with no reported toxicity. Of the 5 patients treated, flattening was noted in one injected tumor nodule, and tumor cell necrosis was evident in the lesions of 3 other patients. HSV was only detected in the malignant cells . Another phase I multiorgan solid tumor (breast, head and neck, gastrointestinal, and metastatic melanoma) clinical trial using JS1/ICP34.5-/ICP47-/GM-CSF demonstrated an acceptable safety profile, with responses most frequently occurring in the metastatic melanoma population. Thirteen patients in the single-dose group received escalating doses of vaccine at 10 6 , 10 7 , and 10 8 PFU, with 3 melanoma patients demonstrating a transient flattening in their lesions. Seventeen patients in the multidose group received escalating doses, with 2 patients achieving stable disease. Only 1 other patient in either group had stable disease (breast cancer), and no partial or complete responses were noted. Other patients with melanoma had regression of the injected lesion but progression of disease at other sites or appearance of new lesions. Many patients in this study demonstrated HSV antigen-associated tumor necrosis at the injected sites, and these sites had either no further progression of disease or showed flattening of the lesion .
Given the responses in the phase I trial, a phase II trial was conducted specifically in patients with stage IIIC and IV melanoma with the new-generation vaccine JS1/ICP34.5-/ICP47-/GM-CSF. The primary endpoint of this study was overall response rate by Response Evaluation Criteria in Solid Tumors (RECIST) criteria, with secondary endpoints of median and overall survival as well as safety. Overall response rate was modified to allow progression before response because delayed responses have been noted in other immunotherapeutic agents, such as the U.S. Food and Drug Administration (FDA)-approved anti-CTLA-4 monoclonal antibody (ipilimumab). In addition, biopsies were obtained to confirm complete response .
Fifty patients were enrolled in the study (40 of whom had stage IV disease) and followed for a median of 18 months. Complete responses (CRs) were noted in 8 patients, partial responses (PRs) in 5 patients, and stable disease (SD) in 10 patients. Of the patients with a PR, 2 achieved a surgical CR following resection of a limited number of lesions. Notably, six of the CRs and three of the PRs were in patients with stage IV disease. Regression of disease at noninjected sites was demonstrated in the lymph nodes, liver, and pancreas. Treatment extensions were granted to 4 responding patients: 2 achieved CR by 24 months, 1 achieved a surgical CR at 13 months, and 1 was maintained with ongoing therapy at the time of publication . Overall, 20% of trial patients achieved a nonsurgical CR and 26% of patients achieved a CR, with 3 of these patients having surgical resections of residual disease. The 1-year survival rate for the 15 patients with a PR, CR, or surgical CR was 93%, and the median survival for all patients was 16 months .
Recently, a phase III multicentered, 2:1 randomized controlled study comparing TVEC to recombinant GM-CSF was completed, but final results are not yet available for this study. Patients with measurable and injectable (subcutaneous or lymph node) stage III (b and c) and stage IV M1 (a–c) melanoma were eligible for enrollment. Adult patients with an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1, life expectancy of greater than 4 months from randomization, and organ function preservation were included. Patients with greater than three non-lung visceral metastases or active cerebral or bone metastases were excluded, as were patients with open herpetic lesions. The primary endpoint of this study is the durable objective (CR and PR) response rate lasting 6 months or greater. Preliminary data demonstrated a significant benefit for the patients treated with vaccine compared to GM-CSF (16 vs. 2%). Overall survival is a secondary endpoint, and data are not yet available. A summary of all melanoma clinical trials to date is provided in Table 15.2 .
| Disease | Study | Phase | Viral Strain | N | Efficacy | |||
|---|---|---|---|---|---|---|---|---|
| CR | PR | SD | Description | |||||
| Breast, gastrointestinal, head and neck, and malignant melanoma | Hu et al. | I | JS1/34.5-/47-/GM-CSF | 30 | 0 | 0 | 3 |
|
| Stage IV metastatic melanoma | MacKie et al. | I | HSV1716 | 5 | N/A | N/A | N/A |
|
| Stage IIIC/IV metastatic melanoma | Senzer et al. | II | JS1/34.5-/47-/GM-CSF | 50 | 8 | 5 | 10 | Overall patient survival: 58% at 1 year, 52% at 2 years |
| Metastatic melanoma | Amgen | III | JS1/34.5-/47-/GM-CSF | 439 | Clinical trial completed in June 2011 with primary endpoint of DRR met and overall survival data expected in mid-2013 | |||
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree