Chapter 7
Modeling the Milk-Ejection Reflex
Gareth Leng and Jianfeng Feng
Centre for Integrative Physiology, University of Edinburgh, Edinburgh, UK
Department of Computer Science, University of Warwick, UK
When babies suckle at their mother’s breast, they are rewarded with a let-down of milk that results from the secretion of the hormone oxytocin (video 1). Oxytocin is synthesized by magnocellular neurons in the hypothalamus. Each of these cells has one axon that projects into the neurohypophysis where it gives rise to about 2 000 nerve endings. Oxytocin is secreted from these in response to action potentials (spikes) generated in the cell bodies and propagated down the axons. Normally, spikes are infrequent and asynchronous, but during suckling, every few minutes, each cell fires a burst of spikes that results in the secretion of a large pulse of oxytocin into the bloodstream. This milk-ejection reflex involves a mechanism that affects the activity of the oxytocin cells, and that a negative feedback that “spaces” the bursts. These involve the dendrites of oxytocin cells. Dendrites are not only sites where neurons receive most of their afferent inputs, but are also the sites of release of factors that influence neuronal excitability. Dendritic oxytocin release has both autocrine effects (on the cell of origin) and paracrine effects (on adjacent cells); it can occur not only in response to spike activity, but can also be triggered independently of spike activity, by stimuli that mobilize intracellular Ca2+ stores. Here, we show how synchronized bursting can arise in a neuronal network model that incorporates these features.
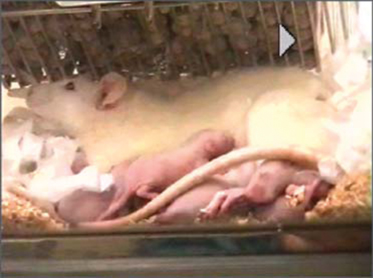
Video 1 The milk-ejection reflex in conscious rats.
7.1 The milk-ejection reflex
In all mammals, oxytocin is made in a few thousand magnocellular neurons whose cell bodies mostly lie within the supraoptic nuclei and the paraventricular nuclei of the hypothalamus. Each of these cells has just one axon; this axon extends into the neurohypophysis, giving rise to about 2000 swellings and nerve endings, each of which is packed with neurosecretory vesicles that contain oxytocin. Spikes that are generated in the cell bodies of the oxytocin cells and that are propagated down the axons cause some vesicles to fuse with the plasma membrane (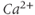 -dependent exocytosis) and release their contents, which then enter the blood. Normally, oxytocin cells fire 1–3 spikes/s, but during suckling, every 5 min or so they all discharge a burst of 50–150 spikes in 1–3 s. These bursts result in the secretion of a pulse of oxytocin that reaches the mammary gland a few seconds later, where it causes milk to release (let down) into a collecting duct from which it can be extracted by suckling.
-dependent exocytosis) and release their contents, which then enter the blood. Normally, oxytocin cells fire 1–3 spikes/s, but during suckling, every 5 min or so they all discharge a burst of 50–150 spikes in 1–3 s. These bursts result in the secretion of a pulse of oxytocin that reaches the mammary gland a few seconds later, where it causes milk to release (let down) into a collecting duct from which it can be extracted by suckling.
The background spike activity of oxytocin cells in lactating rats is very similar to that in nonlactating rats; the cells fire slowly, asynchronously, and nearly randomly. At first, suckling produces little change in this activity, except that slowly firing cells tend to speed up slightly, while faster firing cells slow down. However, after a few minutes of suckling, the first bursts occur. The first bursts are small and involve only some cells, but progressively more and more cells are recruited, until all show intense bursts. Bursts are elicited specifically by the suckling stimulus; many other stimuli cause oxytocin secretion, but they produce a graded increase in electrical activity that is identical in lactating and nonlactating rats, and which does not entail bursting. The bursts (Figure 7.1) differ in amplitude from cell to cell and according to how many pups are suckling, but they are quite consistent in their shape, especially from one burst to the next in a given cell.

Figure 7.1 Milk-ejection bursts. Magnocellular oxytocin neurons each have one axon that projects into the neurohypophysis from where oxytocin is secreted into the general circulation. During suckling, they display intermittent high-frequency bursts of spikes every few minutes. An example of one bursts is shown – the trace is a 3-s extract from an extracellular recording.
7.1.1 The supraoptic nucleus
The hypothalamus contains two supraoptic nuclei: one at the base of the brain and another adjacent to the optic chiasm on either side. Each nucleus contains about 2000 oxytocin cells (Figure 7.2); in addition to its axon, each cell has 2–5 dendrites, and each dendrite contains more than 10000 vesicles. The cells intercommunicate within “bundles” of 3–8 dendrites; in lactating rats, bundles are separated from each other by glial cell processes (thin, sheet-like processes that “wrap round” the bundles), but within each bundle dendrites are directly apposed to one another. In basal conditions, dendritic oxytocin release is not much influenced by spike activity, but it can be evoked by stimuli that mobilize intracellular 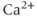 stores. When oxytocin is released from dendrites, it depolarizes oxytocin cells and also mobilizes intracellular
stores. When oxytocin is released from dendrites, it depolarizes oxytocin cells and also mobilizes intracellular 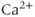 , promoting further oxytocin release.
, promoting further oxytocin release.
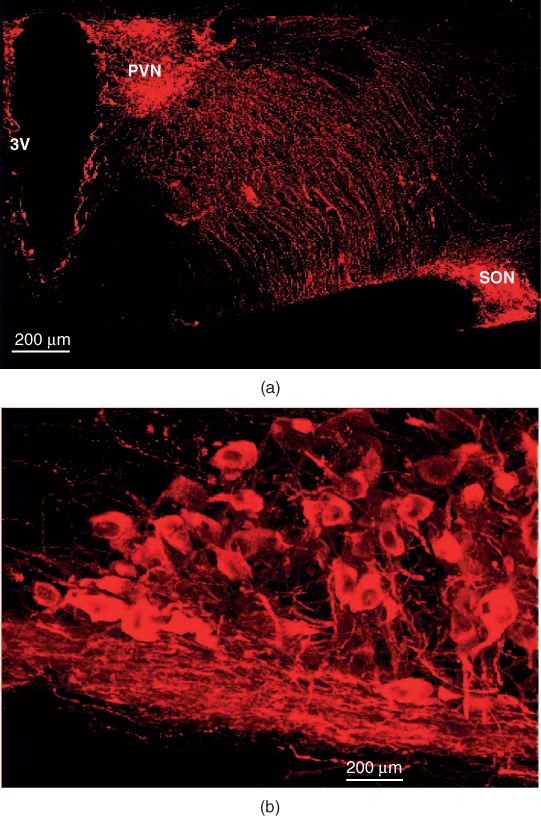
Figure 7.2 The supraoptic nucleus (SON) of the rat hypothalamus. (a) Oxytocin cells in the SON and paraventricular nucleus (PVN) are stained red by immunohistochemistry, in a coronal section of the rat brain. 3V=third ventricle. (b) Higher power view of the SON – the mat of fibers at the base of the nucleus are dendrites. Figure courtesy of Vicky Tobin.
7.1.2 Priming
Mobalization of intracellular  can “prime” the dendritic stores of oxytocin, making them available for subsequent activity-dependent release (by relocating them to sites adjacent to the plasma membrane where they can be influenced by voltage-gated
can “prime” the dendritic stores of oxytocin, making them available for subsequent activity-dependent release (by relocating them to sites adjacent to the plasma membrane where they can be influenced by voltage-gated  entry to fuse with the plasma membrane; Figure 7.3). During suckling, dendritic oxytocin release has been detected before any increase in the spike activity of oxytocin cells, and before any increase in secretion into the blood, so it seems that the suckling input initiates dendritic oxytocin release independently of effects on spike activity. Oxytocin itself is able to prime dendritic stores of oxytocin, so it seems that the suckling stimulus primes the dendritic stores, either purely as a result of evoking oxytocin release or possibly also independently.
entry to fuse with the plasma membrane; Figure 7.3). During suckling, dendritic oxytocin release has been detected before any increase in the spike activity of oxytocin cells, and before any increase in secretion into the blood, so it seems that the suckling input initiates dendritic oxytocin release independently of effects on spike activity. Oxytocin itself is able to prime dendritic stores of oxytocin, so it seems that the suckling stimulus primes the dendritic stores, either purely as a result of evoking oxytocin release or possibly also independently.
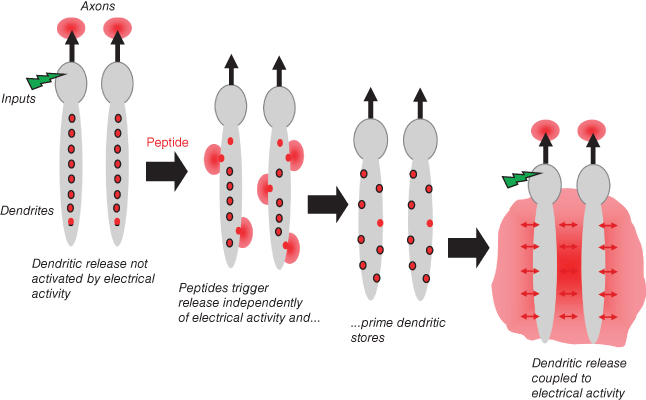
Figure 7.3 Priming in oxytocin cells. The dendrites of oxytocin cells contain many vesicles (shown as red organelles). These vesicles are normally located away from the plasma membrane, so stimuli that increase spike activity (indicated as a green stimulus) trigger release of oxytocin from axon terminals (where many vesicles are located adjacent to the plasama membrane) but not from dendrites. Some peptides can cause release from the dendrites without increasing spike activity, by triggering a mobilisation of intracellular calcium release. In addition, some peptides can prime the dendritic stores – moving vesicles close to the plasma membrane. After priming, these vesicles are available for release in response to increases in spike activity.
7.1.3 Endocannabinoids
Oxytocin cells modulate their afferent inputs by producing endocannabinoids (and other substances), which inhibit excitatory inputs presynaptically, and oxytocin itself suppresses inhibitory inputs by attenuating the effects of GABA. Endocannabinoid production is activity dependent, and is linked to increases in intracellular 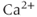 concentration. Endocannabinoids act via specific cannabinoid receptors that are located on afferent endings; oxytocin acts via specific oxytocin receptors which are expressed by oxytocin cells themselves.
concentration. Endocannabinoids act via specific cannabinoid receptors that are located on afferent endings; oxytocin acts via specific oxytocin receptors which are expressed by oxytocin cells themselves.
7.2 The Model
Mathematical modeling involves:
- translating biological statements into differential equations or computational algorithms;
- simulating a biological system by running these equations on a computer to generate “data” that can be compared with observational data;
- “fitting” the model to observations by varying its parameters to ensure that the model data matche in vivo data;
- “testing” the model by using it to generate new predictions or insights.
- simulating a biological system by running these equations on a computer to generate “data” that can be compared with observational data;
In our model of the milk-ejection reflex, each model cell is a modified leaky integrate-and-fire model (Figure 7.4), sometimes called a spike-response model. Such models describe a system that translates synaptic input (transient perturbations of voltage) into spikes by a threshold function. They integrate synaptic inputs over time, calculating the cumulative balance of excitation and inhibition as deviations from a resting potential. A leaky model represents these perturbations as decaying toward the resting potential. A spike arises when the balance of input exceeds a spike threshold. A modified model, or spike-response model, incorporates activity-dependent changes in excitability to mimic the effects of slow voltage and 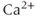 -dependent conductances; these may, for example, mimic hyperpolarizing- or depolarizing-after potentials that follow spikes.
-dependent conductances; these may, for example, mimic hyperpolarizing- or depolarizing-after potentials that follow spikes.
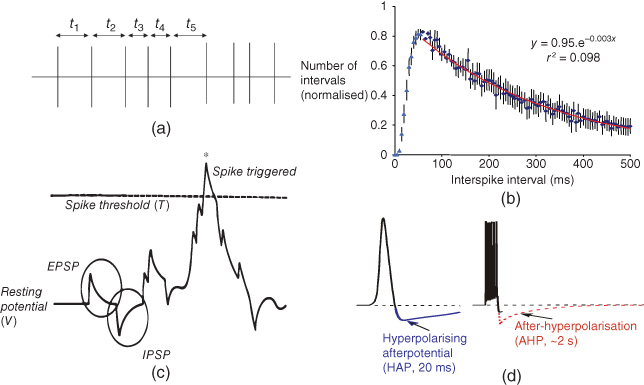
Figure 7.4 Spike activity in oxytocin cells. Under background conditions, oxytocin cells discharge spikes at 1–3 spikes/s. This spiking can be characterized by measuring interspike intervals (t1, t2, etc. as shown in (a)), and constructing an interspike interval histogram. (b) Such histograms have a characteristic distribution tails of the histogram (for intervals 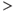 50 ms) that can be well fitted by a single negative exponential (red line, fitted to average of 30 cells). From this, it appears that, after a spike, oxytocin cells have a relative refractory period of about 50 ms, after which spikes arise approximately randomly. In the model, (c) spikes arise in model cells when incoming random EPSPs and IPSPs cause a fluctuation in resting potential sufficient to exceed a spike threshold. The relative refractoriness of oxytocin cells is the result of two post-spike hyperpolarizing mechanisms and (d) a short but large HAP, and a smaller but longer acting AHP (which has a major effect only after bursts). In the model, these two mechanisms are modeled as transient changes in spike threshold that occur after each spike, rather than as changes in the membrane potential – this is equivalent to changes in the membrane potential, but computationally simpler to implement.
50 ms) that can be well fitted by a single negative exponential (red line, fitted to average of 30 cells). From this, it appears that, after a spike, oxytocin cells have a relative refractory period of about 50 ms, after which spikes arise approximately randomly. In the model, (c) spikes arise in model cells when incoming random EPSPs and IPSPs cause a fluctuation in resting potential sufficient to exceed a spike threshold. The relative refractoriness of oxytocin cells is the result of two post-spike hyperpolarizing mechanisms and (d) a short but large HAP, and a smaller but longer acting AHP (which has a major effect only after bursts). In the model, these two mechanisms are modeled as transient changes in spike threshold that occur after each spike, rather than as changes in the membrane potential – this is equivalent to changes in the membrane potential, but computationally simpler to implement.
In this model, every oxytocin cell receives its own, random synaptic input. This is modeled as stochastic excitatory and inhibitory postsynaptic potentials (EPSPs and IPSPs, with realistic reversal potentials); in the model, this input is (normally) balanced, reflecting an equal average mixture of EPSPs and IPSPs (the cells receive an approximately balanced synaptic input, mainly involving the neurotransmitters glutamate and GABA). The resting potential and spike threshold are fixed according to measurements made in vitro, and the size and time course of EPSPs and IPSPs also match observations made in vitro. In the model, these inputs are not directly affected by suckling; they simply ensure that, under basal conditions, each cell has a different, irregular, level of background spiking activity.
7.2.1 Activity-dependent effects on excitability
After every spike, oxytocin cells are refractory because of a hyperpolarizing afterpotential (HAP) that results from a 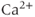 -dependent
-dependent 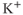 conductance, and which follows spikes in oxytocin cells (because spikes activate high-threshold voltage-activated
conductance, and which follows spikes in oxytocin cells (because spikes activate high-threshold voltage-activated 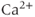 channels). This is modeled as a transient rise in spike threshold, and this alone is sufficient for reproducing the characteristic distribution of interspike intervals observed for oxytocin cells in vivo.
channels). This is modeled as a transient rise in spike threshold, and this alone is sufficient for reproducing the characteristic distribution of interspike intervals observed for oxytocin cells in vivo.
Another modification mimics, the effect of a slower activity-dependent afterhyperpolarization (AHP). This is another 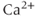 -dependent
-dependent 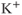 conductance; it mediates a prolonged reduction in excitability after intense activation, and it is enhanced in oxytocin cells during lactation. Including this mechanism enables the model to fully reproduce the shape of milk-ejection bursts.
conductance; it mediates a prolonged reduction in excitability after intense activation, and it is enhanced in oxytocin cells during lactation. Including this mechanism enables the model to fully reproduce the shape of milk-ejection bursts.
7.2.2 Dendritic oxytocin release
Oxytocin secretion from the neurohypophysis is known to be facilitated at high spike frequencies. We assume that activity-dependent dendritic release is similarly nonlinear, and so allow that dendritic oxytocin release only occurs when spikes occur with an interspike interval that is less than a critical value.
How much oxytocin is released also depends on how much is available for release. In dendrites, only vesicles close to the plasma membrane (and hence close to voltage-gated 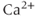 channels) are released by spikes. This readily releasable pool of vesicles is depleted when oxytocin is released and is replenished during suckling – the priming effect.
channels) are released by spikes. This readily releasable pool of vesicles is depleted when oxytocin is released and is replenished during suckling – the priming effect.
7.2.3 Dendro-dendritic communication
Oxytocin cells communicate with each other via their dendrites. Each model cell is given two dendrites, each of which is part of a bundle that includes dendrites from other cells. Dendro-dendritic interactions are modeled by elements that mimic the excitatory effects of oxytocin. This is implemented as an activity-dependent reduction in the spike threshold that affects all of the oxytocin cells that have dendrites in the bundle where oxytocin is released. In the model, recognizing that receptor-mediated effects are subject to saturation, the depolarizing effect of oxytocin is limited to a maximum of 25 mV.
7.2.4 Endocannabinoid release
Oxytocin release is accompanied by the production of endocannabinoids which feed back to modulate synaptic input. Endocannabinoids are produced as a consequence of the mobilization of intracellular 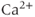 , and act via CB1 receptors on afferent nerve terminals. The rate of release of both EPSPs and IPSPs to all cells connected to a bundle is inhibited by the effects of endocannabinoids produced in that bundle.
, and act via CB1 receptors on afferent nerve terminals. The rate of release of both EPSPs and IPSPs to all cells connected to a bundle is inhibited by the effects of endocannabinoids produced in that bundle.
7.3 Building the model
To model individual cells, we use a leaky integrate-and-fire model (Figure 7.4), which is modified to incorporate activity-dependent changes in excitability. Every cell receives an independent synaptic input that is a mixture of EPSPs and IPSPs, and these are represented by 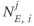 ,
, 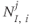 , which are inhomogeneous Poisson processes of rates
, which are inhomogeneous Poisson processes of rates 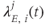 and
and 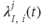 , respectively.
, respectively. 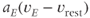 and
and 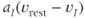 are the magnitude of single EPSPs and IPSPs at
are the magnitude of single EPSPs and IPSPs at 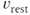 , and
, and 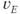 and
and 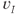 are the excitatory and inhibitory reversal potentials.
are the excitatory and inhibitory reversal potentials.
7.3.1 Spike generation
The membrane potential 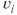 of cell
of cell 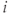 obeys
obeys
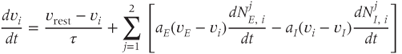
where 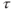 is the membrane time constant, and
is the membrane time constant, and 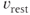 is the resting potential.
is the resting potential.
A spike is produced in cell 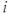 at time
at time 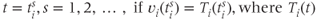 is the spike threshold at time
is the spike threshold at time 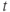 . After each spike,
. After each spike, 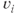 is reset to
is reset to 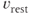 . Activity-dependent changes in excitability and the effects of oxytocin are modeled by effects on spike threshold:
. Activity-dependent changes in excitability and the effects of oxytocin are modeled by effects on spike threshold:
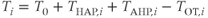
where 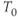 is a constant.
is a constant.
 models the effect of an HAP in cell
models the effect of an HAP in cell 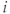 by
by
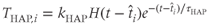
where 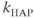 and
and  are constants,
are constants, 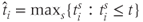 , and
, and 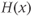 is the Heaviside step function. This gives an increase in the spike threshold after each spike. Similarly,
is the Heaviside step function. This gives an increase in the spike threshold after each spike. Similarly, 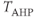 models the AHP. The AHP builds up slowly, leading to a significant reduction of excitability only after intense activity. The variables
models the AHP. The AHP builds up slowly, leading to a significant reduction of excitability only after intense activity. The variables 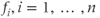 represent the recent activity of each cell, and
represent the recent activity of each cell, and
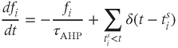
where  is the decay constant of the AHP, and
is the decay constant of the AHP, and 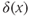 is the Dirac delta function. We set
is the Dirac delta function. We set
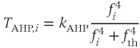
where 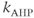 and
and 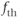 are the constants adjusted to match the characteristics of spontaneous firing in oxytocin cells.
are the constants adjusted to match the characteristics of spontaneous firing in oxytocin cells.
7.3.2 Effects of oxytocin
The network topology (Figure 7.5) – how the cells are interconnected – is represented by matrices 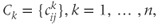 where
where 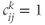 if dendrite
if dendrite 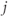 of cell
of cell 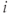 is in bundle
is in bundle 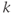 , and zero otherwise. The increase in excitability due to oxytocin is
, and zero otherwise. The increase in excitability due to oxytocin is 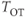 ,
,
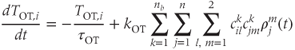
where 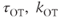 are constants,
are constants, 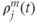 is the release rate from dendrite
is the release rate from dendrite 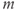 of cell
of cell 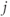 , and the sums pick up all the cells whose dendrites share the same bundle as cell
, and the sums pick up all the cells whose dendrites share the same bundle as cell 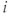 . The oxytocin-dependent reduction of the spike threshold is limited to a maximum (
. The oxytocin-dependent reduction of the spike threshold is limited to a maximum (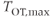 ) of 25 mV.
) of 25 mV.
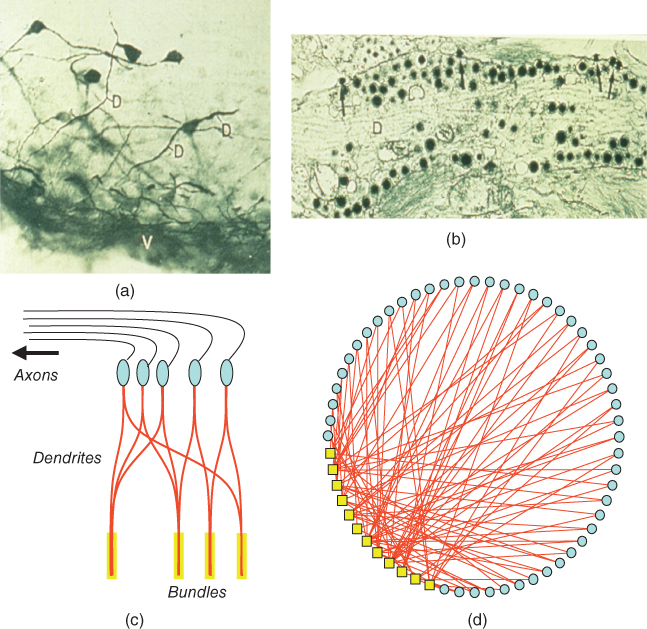
Figure 7.5 Structure of the model network. Oxytocin cells in the supraoptic nucleus have 1–3 large dendrites, most of which project ventrally (shown by immunocytochemistry in (a). These dendrites contain large numbers of neurosecretory vesicles (shown by electron microscopy in (b)). In the model, cells (c, in blue) have two dendrites (in red) that are coupled within bundles (indicated in yellow). The organization of the oxytocin network is shown in (d); the yellow boxes represent dendritic bundles.
7.3.3 Oxytocin release from the dendrites
The readily releasable pool of oxytocin in dendrite 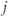 of cell
of cell 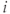 is
is 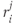 , where
, where
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



