Introduction
Opioid use disorder (OUD) can lead to injection drug use, which is an important risk factor for blood-borne viruses such as HIV and hepatitis C virus (HCV), among other infections. Indeed, the burden of blood-borne viruses among people who inject drugs (PWID) is high globally. A systematic review and meta-analysis [ ] found that 17.8% (95% uncertainty interval [UI], 10.8–24.8) of PWID are living with HIV, 52.3% (95% UI, 42.4–62.1) are HCV-antibody positive, and 9.1% (95% UI, 5.1–13.2) are hepatitis B virus (HBV) surface antigen positive. This equates to an estimated 2.8 million (1.5–4.5 million) PWID living with HIV, 8.2 million (4.7–12.4 million) PWID living with HCV antibody, and 1.4 million (0.7–2.4 million) PWID living with chronic HBV infection [ ]. Among PWID in the United States, an estimated 8.7% (95% UI, 6.8%–10.7%) are living with HIV, 53.1% (95% UI, 38.1%–68.0%) are HCV-antibody positive, and 4.8% (95% UI, 3.0%–7.2%) are HBV surface antigen positive [ ].
Fortunately, there is good empirical evidence from systematic reviews and meta-analyses that harm reduction interventions such as opiate agonist therapy (OAT) and needle and syringe programs (NSPs) can prevent the acquisition of HIV and HCV among PWID, particularly if provided in combination [ ].
Despite this, coverage of harm reduction is low in the United States and worldwide. A systematic review and meta-analysis [ ] found that globally there are 33 [ ] needle-syringes distributed via the NSP per PWID each year, and only 16 [ , ] OAT recipients per 100 PWID. These levels are far below the World Health Organization recommended levels of coverage, defined as >200 needle-syringes distributed per PWID and >40 OAT recipients per 100 PWID. In the United States, an estimated only 30 [ , ] needle-syringes were distributed per PWID per year, and there are only 19 [ , ] OAT recipients per 100 PWID [ ]. Indeed, only four countries (Australia, Austria, the Netherlands, and Norway) have achieved a high coverage of both NSP and OAT. As a result, only an estimated 1% of PWID live in countries with a high coverage of both NSP and OAT [ ].
Mathematical or epidemic modeling has been used extensively to explore and understand infectious disease epidemics among PWID worldwide. In the United States specifically, several epidemic modeling studies have examined HIV and HCV epidemic dynamics among PWID in both urban [ , ] and rural settings [ , ]. Through these studies, modeling has been used to understand important drivers of the epidemic and to identify priorities for prevention.
Modeling can also be used as a “virtual laboratory” to evaluate the population impact of existing harm reduction strategies. Often the evaluation of the impact of existing interventions such as NSPs and OAT at a population level is difficult using epidemiologic data only. For example, natural epidemic expansion or decline could mask or inflate the impact of intervention scale-up, respectively, in the absence of a counterfactual theories, which can be generated through modeling. Additionally, the presence of multiple interventions often makes disentangling the individual impact of each intervention component difficult. Finally, changes in incidence could also be a result of background changes in terms of injecting drug use epidemiology (initiation, cessation, or risk) instead of intervention effect. As a result, modeling studies are useful tools to generate counterfactual theories of the likely epidemic in the absence of the intervention and to disentangle the impact of epidemiologic changes and interventions on the epidemic.
Additionally, modeling can be used for forecasting—to predict the population-level impact of interventions such as harm reduction on disease epidemics and infectious disease transmission. Indeed, modeling has been used extensively to estimate the potential impact of harm reduction interventions, both alone and in combination with other interventions such as HIV and HCV treatment on HIV and HCV incidence and mortality among PWID [ ]. Modeling provides a virtual test laboratory in which different intervention scenarios can be investigated and compared in lieu of implementing large community randomized controlled trials or in preparation for these trials. This is particularly important, because depending on the epidemic stage, the structure of injecting networks, and the injecting practices in a specific population, small increases in intervention coverage might result in large reductions in incidence, whereas in different populations, much higher coverage levels might be required [ ]. In general, these modeling studies have underscored the substantial population prevention benefits that harm reduction, particularly in combination with other treatment and prevention modalities, can play in terms of reducing HIV and HCV incidence among PWID.
There are a broad range of methodological approaches used to evaluate the impact of harm reduction on infectious disease epidemics. Traditional “static” models evaluating the impact of harm reduction, such as OAT, on drug use and overdose incorporate only individual health benefits, whereas more complex “ dynamic” transmission models mechanistically simulate the impact of OAT on associated infectious disease incidence at a population level. These dynamic transmission models therefore incorporate both individual and population prevention benefits and can be either deterministic (compartmentalizing the population into different groups and infection states, assuming the behavior is heterogeneous within each compartment) or individual based (simulating each individual separately within each population). The selection of model type (e.g., deterministic vs. individual based) is often based on the research question and available data. Models evaluating the impact of NSPs provision have generally fallen into two categories: models that utilize relatively simple calculations of change in infection incidence over 1 year predicted by a change in the population proportion of contaminated syringes circulating among PWID (entitled “circulation theory” models) and dynamic transmission models that assess long-term impact and account for the entire future chain of infections averted. As stronger systematic review data emerges on the impact of harm reduction interventions on an individual’s risk of HIV and HCV incidence, more robust evaluations can occur estimating the short- and long-term impact of harm reduction on related infectious disease epidemics.
This chapter reviews the real-world and modeling evidence surrounding the impact and cost-effectiveness of harm reduction for OUD in the prevention of infectious diseases, with a focus on analyses for the United States.
Empirical evidence for harm reduction on acquiring HIV and hepatitis C virus infections
Harm reduction interventions for people who use and inject drugs include medication-assisted treatment and NSPs. Medication-assisted treatment includes OAT, with drugs such as methadone and buprenorphine, and opioid antagonist treatment, with drugs such as naltrexone. It is well known that OAT is effective in reducing fatal overdose. Evidence from a global meta-analysis of 21 cohorts of nearly 140,000 patients who underwent OAT found that methadone reduced the risk of overdose by 79% (relative risk [RR] 0.21; 95% CI, 0.13–0.34) [ ]. Only one cohort study was conducted among patients who used buprenorphine, but the magnitude of the effect on fatal overdose prevention was similar (approximately 70% reduction) [ ]. Two global systematic reviews and meta-analyses have found strong evidence that OAT reduces an individual’s risk of acquiring HIV by 54% (RR 0.46; 95% CI, 0.32–0.67) [ ] and acquiring HCV by 49% (RR 0.51; 95% CI, 0.4–0.63) [ ]. The results were consistent across geographic regions. For example, the effect estimates in North America were similar to the overall pooled effect, with OAT reducing an individual’s risk of acquiring HIV by 62% (RR 0.38; 95% CI, 0.23–0.65) [ ] and HCV by 43% (RR 0.57; 95% CI, 0.42–0.77) [ ]. Evidence of the effectiveness of antagonist treatments such as naltrexone on reducing HIV and HCV acquisition risk is mixed, and therefore will not be discussed here.
NSPs provide sterile needles, syringes, injecting equipment, education, and other prevention and support services. There is evidence that NSPs not only reduce injecting risk behaviors but also prevent the acquisition of HIV and HCV. A systematic review found that exposure to NSPs reduces an individual’s risk of acquiring HIV by 44% (RR 0.6; 95% CI, 0.43–1.01) across all studies with high heterogeneity, and 0.42 (95% CI, 0.22–0.81) across six higher quality studies [ ]. A Cochrane review [ ] found weaker evidence for NSPs in reducing HCV, with the overall finding that high coverage of NSPs reduces an individual’s risk of acquiring HCV by 23% (RR 0.77; 95% CI, 0.38–1.54) compared to no or low NSPs coverage, and combined OAT and NSPs results in a 76% reduction in HCV acquisition risk (RR 0.24; 95% CI, 0.09–0.62). However, there was high heterogeneity across studies regarding the impact of NSPs on HCV infection and the effect varied by region [ ]. For example, high NSPs coverage in Europe was associated with a 56% reduction in HCV acquisition (RR = 0.44; 95% CI, 0.23–0.8) with low heterogeneity, whereas no effect was found for North America (RR = 1.58; 95% CI, 0.57–4.42), which had high heterogeneity [ ]. There are several potential explanations for this geographic difference in the observed NSPs effect. First, while consistent measures of NSPs exposure through individual-level coverage estimates were available from Europe (measured by receiving one or more sterile syringes for each injection), measures in North American studies varied and focused more on the frequency of attendance at NSP instead of coverage. These inconsistencies in measurement contributed to the difference in heterogeneity between the studies, and these exposure measurement differences combined with differences in study design and injecting patterns may account for a lack of observed effect in North America. Additionally, the lack of federal funding for NSPs during the period the studies were performed has resulted in lower coverage of NSPs among PWID in the United States compared with the European sites, which may mask the intervention effect. Finally, the higher proportion of PWID injecting stimulants in the US studies could contribute to lower impact. Residual confounding may be important, especially in North America, where people who attend NSPs also report higher injection risk behaviors and factors associated with injecting/sexual risk (such as sex work and homelessness) so that adjustment for these factors may attenuate any positive association between HIV or HCV transmission and NSPs attendance.
Supervised injection facilities (SIFs), along with supervised drug consumption rooms more generally, are facilities that provide safer environments for drug use. These sites provide a place for hygienic consumption of drugs under the supervision of trained staff. The primary aim is to reduce unsterile drug use practices, prevent drug-related overdose deaths, and facilitate connections with drug use treatment and health and social services. Typically, SIFs provide sterile injecting equipment, counseling, emergency overdose care, and referral to the aforementioned services. The first SIF was established in 1986 in Switzerland, with subsequent expansion across Europe. Ten countries currently allow legal operation of SIFs (Australia, Canada, Denmark, France, Germany, Luxembourg, the Netherlands, Norway, Spain, and Switzerland). In the United States, the legal status of SIFs was unclear until October 2019, when a District Judge ruled that “the ultimate goal of Safehouse’s proposed operation is to reduce drug use, not facilitate it, and accordingly, §856(a) does not prohibit Safehouse’s proposed conduct”. Documentation of an unsanctioned SIFs in the United States operating since 2014 has been reported [ ]. A review of reviews [ ] indicated that there was “tentative evidence” to support the effectiveness of SIFs in reducing injecting risk behavior and improving injecting hygiene. Although encouraging, authors generally conclude that the evidence to evaluate any impact on HIV and HCV incidence is insufficient and further studies are needed in this area.
In summary, there is strong evidence that OAT reduces overdose, as well as an individual’s risk of acquiring HIV and HCV infection. There is good but weaker evidence that high-coverage NSPs reduce the acquisition of HIV and HCV globally; however, there is high heterogeneity and evidence for stronger effect of high NSP coverage in Europe, but no effect in North America. In combination, there is strong evidence that the receipt of both OAT and high-coverage NSP prevents acquisition of HCV. There is tentative evidence that SIFs reduce injecting risk, but the evidence is insufficient and more studies are needed to evaluate the impact on HIV and HCV incidence.
Modeling the impact of harm reduction on HIV epidemics among people who inject drugs in the United States
Modeling opiate agonist therapy for HIV prevention
For more than 50 years, methadone treatment, a form of OAT, has been prescribed to treat patients with OUD in the United States [ ] and was readily available prior to the emergence of HIV in the early 1980s. In the United States, early work by Zaric et al. [ ] in 2000 used a dynamic transmission model to investigate the impact of a 10% relative increase in methadone coverage in two settings with low (5%) and high (20%) HIV prevalence resembling Los Angeles and New York City, respectively, and found that it would result in 34 and 264 HIV infections averted over 10 years, respectively, in each setting. This analysis assumed a relatively high (94%) reduction in HIV risk on OAT. While updated estimates of methadone effectiveness from global systematic reviews have become available since [ ], this modeling study contributes valuable information to policy makers because it found that 36% and 28% of HIV infections averted in the low- and high-prevalence settings, respectively, would be among the general population, thus showing the benefits of methadone scale-up would go beyond the PWID population [ ]. This information can be a key argument when advocating for the expansion of harm reduction services.
Modeling needle and syringe programs for HIV prevention
Initial studies evaluating the impact of NSPs on HIV epidemics utilized ecologic analyses, comparing HIV prevalence across sites with and without NSPs provision. A 1997 ecologic analysis by Hurley and colleagues [ ] used data from 81 cities across Europe, Asia, and North America, finding that HIV prevalence increased by an average of 5.9% per year in cities without NSPs (n = 52). By contrast, HIV prevalence decreased by an average of 5.8% per year in the cities with NSPs (n = 20). One particularly striking example was evident from New York City, where the introduction of NSPs occurred alongside a concomitant marked decrease of HIV incidence among PWID [ , ]. Nevertheless, ecologic studies are limited because changes in HIV incidence or prevalence could be due to other factors such as background changes in mortality and epidemiology, so disentangling the impact of NSP is difficult.
Numerous early studies utilized a simplified mathematic framework developed by Kaplan [ ] called the needle circulation theory to evaluate the potential impact of NSPs in the United States and the associated cost-effectiveness (described in the section Modeling the impact of harm reduction among incarcerated populations on infectious diseases ). Kaplan’s analysis uses population estimates of the number of injecting events per year and the number of sterile syringes distributed per year by NSPs to generate estimates of the proportion of contaminated syringes circulating in a population. It is then assumed that the more syringes distributed via NSPs, the shorter the time a syringe is in circulation, which lowers the proportion of circulating contaminated syringes and subsequently reduces the associated injecting-related disease incidence. This hypothesis was supported by programmatic data indicating the mean circulation time, as well as the prevalence of HIV within syringes exchanged at a syringe exchange in New Haven, Connecticut, which was reduced across the 20 months of operations from 1990 to 1992 [ ]. With this framework, Kaplan used programmatic data on the number of clients reached and syringes distributed to evaluate the impact of the New Haven SEP, estimating it reduced new HIV infections among attendants by 33% in 1 year [ ]. Additionally, the analysis found that the SEP distributed 168 syringes per PWID per year and contacted 200 PWID, predicting that this distribution averted 1.62 HIV infections per year [ ]. Interestingly, this model predicted that an optimal resource allocation of more syringes among fewer PWID (761 syringes per PWID per year among 165 PWID) could avert 2.5 HIV infections per year [ ]. Another analysis by Laufer et al. [ ] of NSPs in New York utilized a circulation theory as well as estimates of HIV incidence inside and outside NSPs in New York City to estimate the impact of seven NSPs located in New York City and Rochester, New York, in 1992. Using these estimates, these SEPs were predicted to decrease HIV incidence among clients by 60%, resulting in 87–92 HIV infections averted per year.
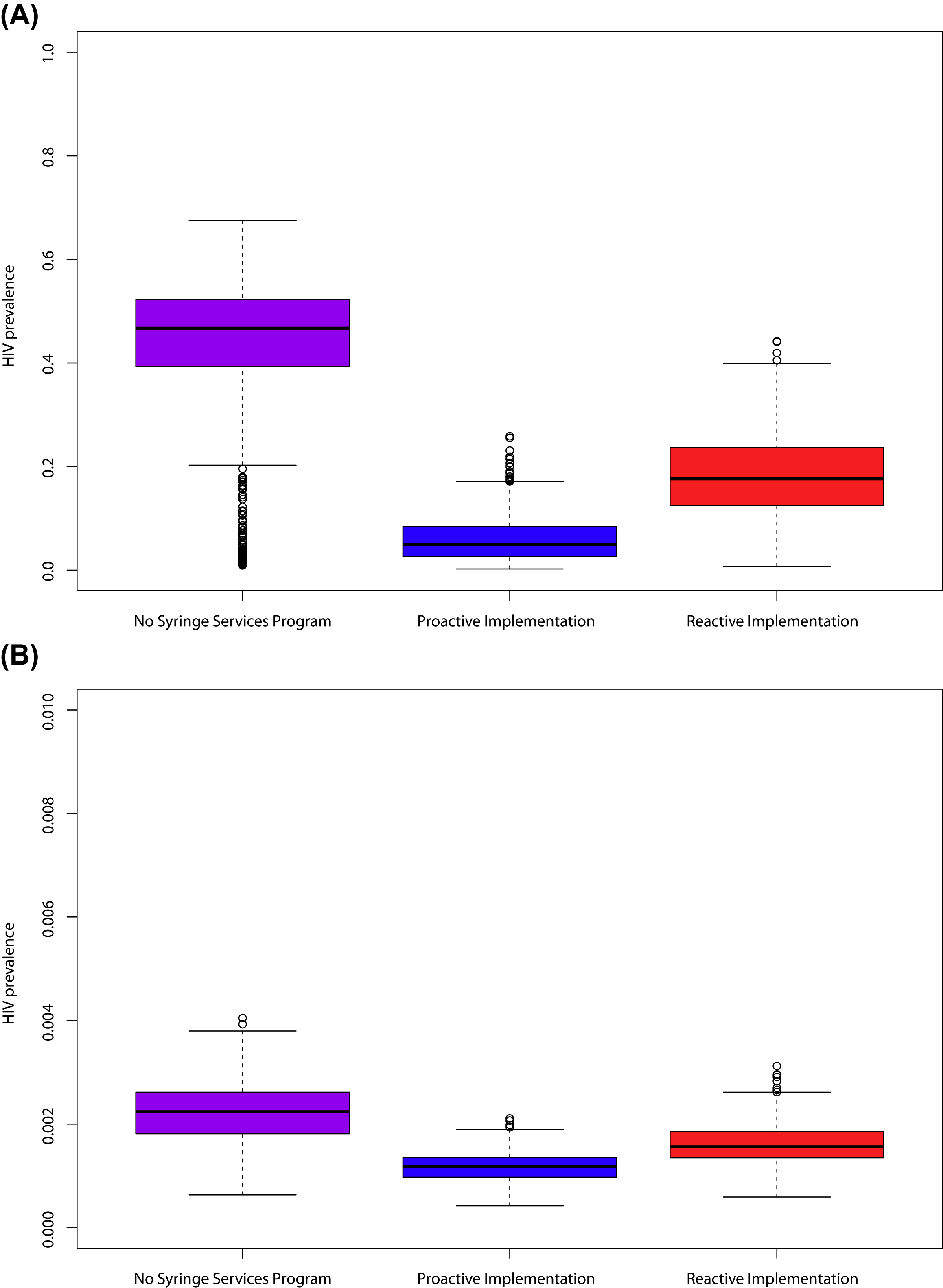
The predicted impact of NSPs varies substantially by epidemiologic setting and assumptions about intervention efficacy, but it generally indicates the marked impact on averting HIV with NSPs scale-up. For example, Lurie and colleagues [ ], using a mixture of circulation theory and dynamic models, simulated four hypothetical cities with different epidemiologic characteristics and predicted that NSPs implementation could result in reductions in HIV incidence among PWID between 17% and 70%. A subsequent analysis by Lurie et al. [ ] estimated the HIV infections associated with a lack of comprehensive NSPs in the United States. They estimated that 4394–9666 HIV infections could have been averted between 1987 and 1995 with a scale-up similar to that achieved in Australia [ ]. An analysis by Nguyen et al. [ ], also based on a circulation theory, estimated that a national investment of an additional $10 or $50 million for NSPs providing a minimal set of services would avert 194 or 816 new HIV infections among PWID over a year in the United States. Finally, an analysis by Geodel et al. used an individual-based dynamic transmission model to examine the impact of barriers to NSPs implementation in a rural US county similar to Scott County, Indiana, after the introduction of a single HIV infection. Using data from Scott County, they simulated the impact of an NSP with 55% attendance among PWID and reductions in self-reported syringe sharing from 34% to 2% among those attending the NSP. Using these assumptions, their analysis found that there would be on average 124 infections within 5 years if no NSP was implemented, whereas reactive implementation of an NSP beginning operations 10 months after the first infection (as occurred in Scott County) would prevent 76 infections. On the other hand, if NSPs were preexisting before the HIV infection introduction, they would avert 105 infections. Importantly, the proactive (preexisting NSP) scenario would result in lower prevalence among both PWID and the broader population, indicating the important community benefit of NSPs ( Fig. 13.1 ). Gonsalves et al. [ ] carried out a similar analysis of the Scott County outbreak using a deterministic model, also finding that the outbreak could have been reduced to ten or fewer infections through early intervention.
Despite these analyses, there remains uncertainty in how the expansion of NSPs translates to changes in individual-level syringe distribution coverage, syringe sharing, and the resulting HIV transmission. An analysis from Australia by Kwon et al. [ ] used data on the dose-response relationship between individual-level syringe distribution coverage and individual self-reported syringe sharing, indicating that in the absence of NSPs the syringe sharing rate would have been 20%–45%, which reduced to <20% with the expansion of NSP in the early 1990s to achieve a coverage of roughly 50 needle-syringes distributed through NSPs per capita per year. However, subsequent increases in the number of needle-syringes distributed through NSPs per capita to above 150 did not substantially reduce sharing further. These data are important in quantifying the benefits of NSP provision and coverage, which may not be linear. Unfortunately, no equivalent data are available for the United States, although the relationship is likely to be similar. Using these data, Kwon estimated that NSPs in Australia reduced the incidence of HIV among PWID by 34%–70% from 2000 to 2010, a dramatic impact with a comprehensive national program.
Modeling combination opioid agonist therapy and needle and syringe program for HIV prevention
In an analysis, Bernard et al. [ ] utilized dynamic transmission modeling to investigate the potential impact of scaled-up combination HIV prevention portfolios including harm reduction on reducing HIV among PWID in the United States. They explored the impact of combinations of OAT, NSP, HIV testing and treatment, and HIV preexposure prophylaxis on HIV among PWID. Bernard and colleagues [ ] found that scaling up OAT or NSP to 50% coverage among PWID could reduce HIV prevalence by a relative 16%–17% at 20 years, which would avert 22,000 or 35,000 HIV infections among PWID over 20 years, respectively. A combination of scaled-up OAT, NSP, HIV test and treat, and HIV preexposure prophylaxis interventions at high coverage (50% of eligible population) would avert 62,000 (95% confidence interval [CI], 37,000–92,000) over 20 years [ ].
Using a model of HIV transmission among PWID in New York City, Marshall et al. [ , ] also investigated the impact of HIV prevention packages incorporating harm reduction. They examined the impact of a 50% relative increase in HIV testing, OAT coverage, NSPs impact on syringe sharing, and treatment as prevention coverage, implemented alone, in combination with another intervention (dual prevention strategy), or as a combination of all interventions (combination prevention), on HIV incidence among PWID. They found that, if implemented in isolation, increased OAT coverage resulted in a 26.3% (95% CI: 11.3%, 41.3%) relative reduction in HIV incidence by 2040 and increased NSPs access resulted in a 34.3% (95% CI: 19.4%, 49.2%) reduction but that neither of these individual strategies reduced HIV incidence to <1 per 1000 person-years by 2030 [ ]. However, joint OAT and NSPs scale-up could result in an HIV incidence among PWID of <1 per 1000 person-years by 2040 [ ]. Furthermore, the full combination prevention strategy resulted in a 62.4% (95% CI: 52.6%, 72.2%) reduction in incidence in 2040 compared to the status quo [ ]. The combination prevention strategies all had higher impact on incidence compared with scenarios in which interventions were implemented alone, highlighting the epidemiologic benefits of a comprehensive combined intervention approach.
These US analyses are consistent with other global modeling studies that have concluded that scaled-up combination prevention including harm reduction could have a substantial impact on both individual acquisition of HIV and population-level transmission among PWID in a wide range of countries and epidemiologic settings [ , , ].
Additionally, several international studies have evaluated the substantial impact that existing combination harm reduction programs have had on HIV epidemics among PWID. For example, two modeling studies have explored the relative impact that scaled-up harm reduction and antiretroviral therapy (ART) efforts could have had on the HIV epidemic among PWID in Vancouver [ , ]. In Vancouver, substantial declines in HIV and HCV incidence among PWID between 1996 and 2013 occurred alongside the expansion of both ART and harm reduction interventions [ ]. In one study, Fraser et al. [ ] used dynamic coinfection modeling and found that the large declines in HCV incidence observed (84% relative decline from 1996 to 2007) required substantial declines in injecting risk (by nearly 60%), which likely accounted for the majority of the observed decline in HIV incidence. As such, the authors conclude that the observed declines in HIV incidence should be seen as a success for intensive harm reduction interventions, whereas ART may have played a smaller role. In a separate analysis, Nosyk et al. [ ] also used modeling to retrospectively investigate the impact and contribution of harm reduction and ART interventions on HIV incidence in British Columbia, Canada. They estimated that their implementation averted 3204 (2402–4589) HIV infections between 1996 and 2013 [ ]. To estimate the maximum impact of harm reduction versus ART interventions, they implemented hypothetical scenarios in which ART had no impact on injecting transmission and in which the coverage of harm reduction had remained constant at 1996 levels. Under such extreme assumptions, 77% (62%–95%) and 44% (10%–67%) of HIV infections averted would be attributable to harm reduction and ART interventions, respectively [ ]. They concluded that even if ART effectiveness on injecting transmission was as high as that for sexual transmission, harm reduction interventions would still have played a key role in lowering HIV incidence.
Modeling supervised injection facilities for HIV prevention
Several analyses have evaluated the population impact of SIFs on HIV among PWID in North America. Although only one government-sanctioned SIF has been functioning in North America for several years (Insite in Vancouver), interest has grown substantially in response to the overdose crisis and a few new SIFs have emerged. Several groups have investigated the effectiveness of Insite in reducing HIV incidence, with estimates varying from 4 to 119 HIV infections per year [ ]. Analyses with lower estimates, such as those by Pinkerton et al. [ , ], did not consider behavior change in terms of sharing rates, whereas other studies incorporated data indicating lower rates of sharing among Insite attendants [ , ]. Additionally, higher estimates of infections averted tended to incorporate dynamic models that include the full chain of onward infections averted [ ]. Other analyses evaluated the potential impact of opening SIFs in Toronto, Ottawa, and Montreal in Canada [ , ]. These analyses estimated that opening one to three SIFs would avert 2–3 HIV infections per facility in Toronto, 6–10 HIV infections per facility in Ottawa, and 11 HIV infections per facility in Montreal over 20 years [ , ]. The impact of opening fourth and fifth facilities on HIV incidence would be considerably less. In the United States, Irwin et al. [ , ] carried out similar analyses to estimate the potential impact of an SIF on HIV incidence in San Francisco and Baltimore, predicting that a single SIF could avert 3.3 and 3.7 HIV infections per year, respectively. Overall, these studies provide important evidence that SIFs can avert HIV infections, but the predicted impact depends on the epidemic setting, assumptions of intervention impact, and the model type.
Modeling hepatitis C virus infection and harm reduction in the United States
Modeling opioid agonist therapy and needle and syringe programs for hepatitis C virus infection prevention
Early theoretic analyses by Pollack et al. [ , ] evaluated the impact of NSPs on steady-state HCV prevalence among PWID in general settings with and without NSPs. These analyses compared methodological approaches and by utilizing dynamic modeling indicated that short-term incidence analyses overstate NSPs effectiveness in preventing HCV and recommended using longer-time time horizons with dynamic approaches [ , ]. More recent analyses evaluating the impact of existing harm reduction interventions on HCV infection incorporated updated effect estimates for NSPs on HCV incidence or observed empirical relationships between syringe coverage and sharing behavior, combined with transmission epidemic modeling. As detailed earlier, Kwon et al. [ , ] utilized observed relationships between individual-level syringe coverage and syringe sharing behavior in Australia and a dynamic transmission model to estimate that NSPs reduced the incidence of HCV infection among PWID by 15%–43% (19,000–77,000 cases) during 2000–10.
Another UK study evaluating the impact of the existing harm reduction provision by Vickerman et al. used surveillance data to estimate the proportion of PWID on OAT and the proportion receiving high-coverage NSPs (defined as receiving one or more sterile syringes for each injection) combined with the effect estimates for these interventions from systematic reviews and a dynamic transmission model. They found that the existing high levels of harm reduction coverage (>50% of each) could have prevented very high prevalence of HCV infection (>65% chronic prevalence among PWID, compared with the 40% observed) [ ]. Another study by Fraser et al. [ ] evaluated the scale-up of harm reduction programs in Scotland associated with government-funded national strategies, which promoted the scale-up of OAT, NSPs, and some increases in HCV treatment from 2008. They used modeling to test whether observed decreases in HCV incidence after 2008 could be attributed to this intervention scale-up and found that scaling up of all interventions averted 1492 (95% credible interval [CrI], 657–2646) infections over 7 years, with the majority due to the scale-up of OAT and NSPs (1016 infections, 95% CrI, 308–1996) [ ].
No recent studies have evaluated the population impact of existing harm reduction provision on HCV infection in the United States, partly because coverage is very low in many settings and additionally because focus has shifted toward how settings can achieve incidence reductions of 80%–90% by 2030, in line with the goals of the World Health Organization [ ] and US National Academies of Sciences, Engineering, and Medicine [ ]. As such, analyses have tended to focus on the potential future impact of scaled-up levels of HCV treatment for both individual cure and population prevention benefit, such as analyses in Chicago, Illinois [ ], and Hartford, Connecticut [ ]. Furthermore, modeling analyses by Martin et al. [ ] across several generalized HCV epidemic settings (20%, 40%, and 60% chronic HCV infection among PWID) indicated that scale-up of harm reduction alone could reduce HCV incidence by up to 40% within a decade, but that further reductions required a combination harm reduction and treatment strategy. Their analyses indicated that combination prevention strategies incorporating both treatment and harm reduction can be highly effective in achieving elimination targets and can reduce the numbers needed to treat.
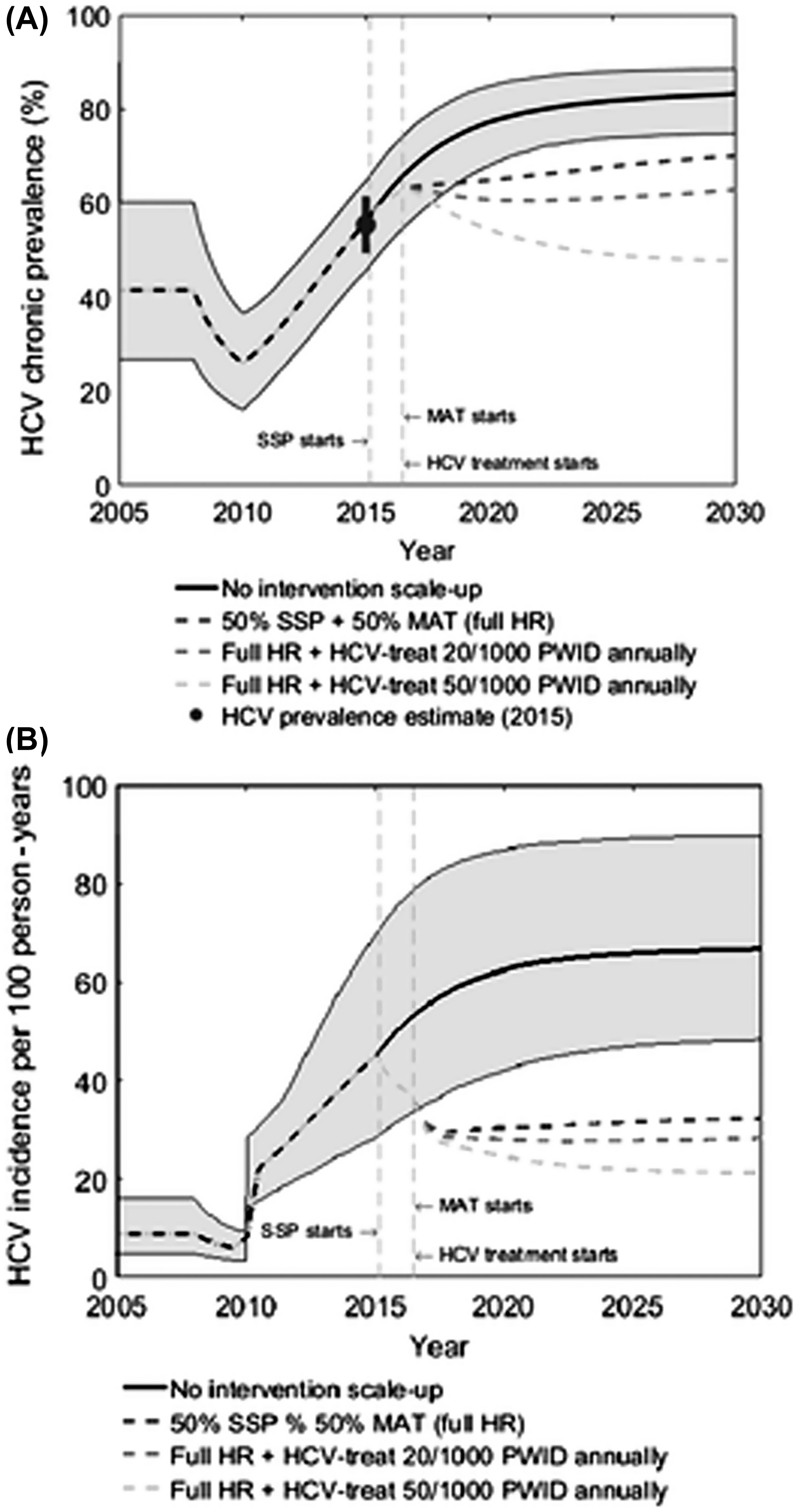
This general work was supported by two recent US-focused studies that assessed what level of scaled-up combination intervention (harm reduction and HCV treatment) could achieve reductions of 90% in HCV incidence by 2030. The first work, by Fraser et al. [ ], examined what is needed in Scott County, Indiana, a rural setting with a rapidly expanding HCV epidemic and increasing injecting drug use. They found that elimination can be achieved through scale-up of HCV treatment only, but that combination scale-up of harm reduction (NSP and OAT to 50% coverage among PWID) halves the treatment rate required. Importantly, they also found that harm reduction provision was essential for prevention of reinfection. If reinfections are not retreated, the epidemic will rebound due to reinfection unless harm reduction is present to sustain treatment impact ( Fig. 13.2 ). This underscores the critical importance of harm reduction as a key component of HCV intervention strategies to prevent both primary infection and reinfection.