Fig. 7.1
Surgeon’s console
Fig. 7.2
Patient cart
Robotic surgery enables surgeons to be more precise, advancing their technique and enhancing their capability in performing complex minimally invasive surgery.
Binocular stereoscopic 3D vision with stability of camera and 10× magnification allows the surgeon better visualization of the anatomy, which is especially critical when working around delicate and confined structures like in the pelvis, chest, or abdomen. This allows surgeons to perform radical cancer surgeries with superior oncological outcome.
It mimics the human hand in its flexible movement and also overcomes limitations of it, like 7° of movement and elimination of hand tremors. Despite the widespread use of laparoscopic surgery, adoption of laparoscopic techniques, for the most part, has been limited to a few routine procedures. This is due mostly to the limited capabilities of traditional laparoscopic technology, including standard video and rigid instruments. Surgeons have been slow to adopt laparoscopy for complex procedures because they generally find that fine-tissue manipulation such as dissecting and suturing to be more difficult. Intuitive technology, however, enables the use of robot for complex procedures. The robot allows for 7° of motion vs. the limited 4° of motion in laparoscopy. Robotic technology eliminates the fulcrum effect of laparoscopy (the robotic arms imitate the movements of the surgeon’s hand).
Motion scaling and precision of surgical movements during robotic surgery improve the quality of surgery. Extremely easy and fast suturing and knotting and multitasking instrumentations decrease operative time. Surgeon sits and operates at ease with less fatigue, translating to safe surgery.
Surgical Technique
Preoperative Preparation
Patient takes clear liquids a day prior to surgery. Proctoclysis enema and two Dulcolax (bisacodyl) tablets are given per oral a night before the surgery. We do not administer Peglec which causes dilatation of the bowel.
Port placement (Figs. 7.3 and 7.4) and instrumentation (Fig. 7.8)
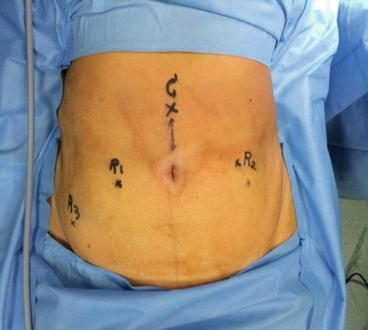
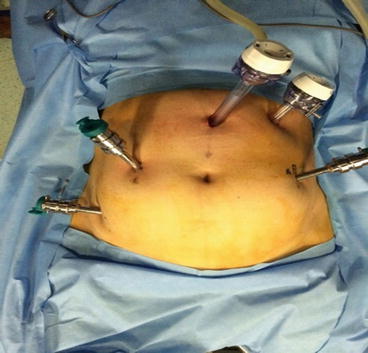

Fig. 7.3
Abdominal marking of port placement

Fig. 7.4
Port placement
Vaginal-Cervical Ahluwalia Retractor-Elevator (VCARE) uterine manipulator is fixed to the cervix after placing patient in lithotomy position. Intraoperatively, it helps in manipulating the uterus. A 12 mm camera port is placed 3 cm above the umbilicus in the midline with optical trocar. The rest of the ports are placed after insufflating the abdomen with gas and marking the port measurements. Arm-one (8 mm) port is placed on patient’s right side, 3–5 cm below and at least 8 cm lateral to the camera port. Arm-two (8 mm) port is placed on patient’s left side, 8 cm lateral and 3–5 cm below the level of the camera port. Arm-three (8 mm) port is placed on patient’s right side, 2 cm above the anterior superior iliac spine and 8 cm away from the first port. Assistant port (12 mm) is placed on patient’s left side, slightly cephalad to the camera port on an arc at the midpoint between the camera port and the instrument arm-two port.
Zero-degree scope is used for all the steps, except for para-aortic lymph node dissection where 30° down scope is used. In arm-one hot shears (monopolar curved scissors), in arm-two fenestrated bipolar forceps, and in arm-three prograsp forceps is used (Figs. 7.5 and 7.6, 7.7).
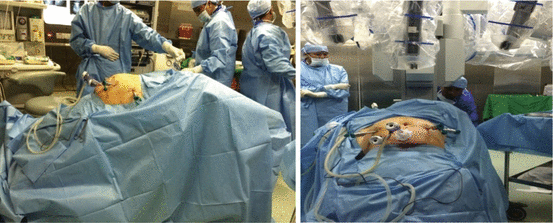

Figs. 7.5 and 7.6
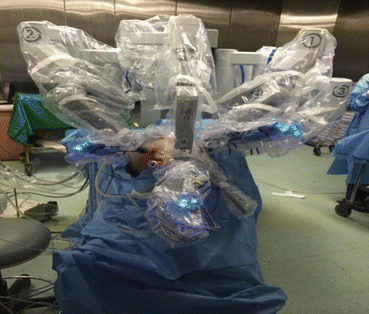
Patient positioning and Docking in progress

Fig. 7.7
Post docking
After placing all the ports, the patient is positioned before docking the robot. Head end side is lowered completely, and all the bowel loops are taken toward the upper abdomen. Pelvic wash is given and fluid is taken for cytological examination (Fig. 7.8).
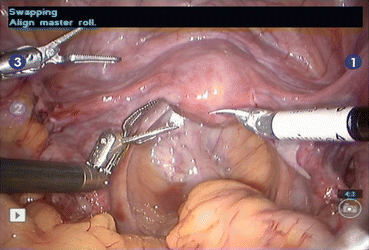

Fig. 7.8
Robotic instruments with endowrist technology
Surgical Steps
Dissection is done in a circular fashion from one round ligament to the other.
Step 1: The uterus is retracted to the patient’s left side with the help of uterine manipulator. Dissection starts with incising the peritoneum over the infundibulopelvic triangle, isolating the ureter and ovarian pedicle. Then, the round ligament is transected near the inguinal ring with hot shear (monopolar diathermy). Incision is extended anteriorly into the anterior leaf of the broad ligament up to the lateral uterovesical junction. Coagulate and transect the right uterine pedicle and cardinal ligament. Pay careful attention to the course of the ureter.
Step 2:The urinary bladder is lifted up with third arm, and the uterus is retroverted with the help of uterine manipulator and second arm. The vesicouterine groove is identified and the bladder is dissected away from the uterus, and adhesions if any are dissected with the cold knife (hot shear).
Step 3: Left-side isolation of the ureter and dissection of the round ligament are done similar to step 1. Both side ovarian pedicles are coagulated with bipolar diathermy but not divided until complete dissection is done.
Step 4: Posterior part dissection is done by separating the rectum from the uterus with the division of the uterosacral ligaments on either side. The course of the ureter must be noted during this step.
Step 5: Anterior and posterior colpotomies are done by incising over the colpotomy ring. Finally, both the ovarian pedicles are divided. Specimen is delivered through the vagina by pulling out the uterine manipulator, and abdominal pneumatic pressure is maintained by packing the vagina with an adequate size ball made of mop inside a surgical hand glove.
Step 6: Bilateral pelvic lymphadenectomy (Figs. 7.11 and 7.12) is done by exposing the pararectal and paravesical spaces. Separate specimen bag is used for each side of the lymph nodes, and specimen is delivered through the vagina. Para-aortic lymph node dissection is done when indicated. The vaginal cuff is closed with a 15 cm long self-retaining polydioxanone (monofilament, violet) barb suture, and uterosacral ligaments are included laterally.
The role of systematic pelvic lymphadenectomy is an issue of current debate. Excision of suspicious or enlarged nodes is important to exclude metastasis. A more selective and tailored lymphadenectomy approach is now recommended to avoid systematic overtreatment [6]. No randomized trial data support full lymphadenectomy [7] although some retrospective studies have suggested that it is beneficial [8]. A subset of patients may not benefit from lymphadenectomy, but it is difficult to preoperatively identify these patients because of the uncontrollable variable of change in grade and depth of invasion in final histopathology.
As the grade of the tumor increases, accuracy of intraoperative evaluation of myometrial invasion by gross examination decreases. Therefore, frozen section examination for evaluation of the histology, size of primary, grade, and depth of invasion is important. Pending further trials, pelvic lymphadenectomy is done in all patients. Para-aortic lymphadenectomy is indicated in high-risk patients.
Anatomical spaces in pelvic dissection:
- 1.
Paravesical space
- 2.
Pararectal space
Anatomical boundaries:
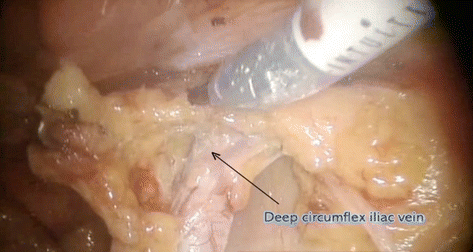
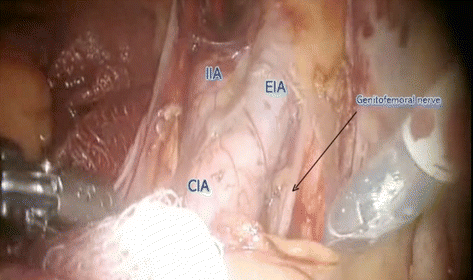
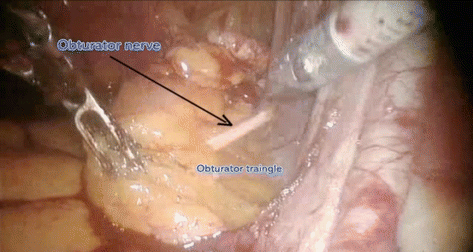
Distal – deep circumflex iliac vein
Proximal – common iliac vessels
Laterally – genitofemoral nerve

Fig. 7.9
Pelvic lymphadenectomy – distal boundary

Fig. 7.10
Pelvic lymphadenectomy – lateral and proximal boundary

Fig. 7.11
Pelvic lymphadenectomy – inferior boundary

Fig. 7.12
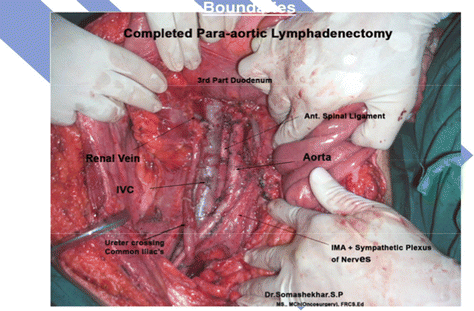
Completed paraortic lymphadenectomy with critical structures
Para-aortic Lymphadenectomy
Boundaries
Superiorly – renal vein
Inferiorly – common iliac vessels
Laterally – ureter
Evolving Evidence
Efficacy of Laparoscopy
The Gynecologic Oncology Group (GOG) has completed a phase III randomized study (lamina-associated polypeptide 2 (LAP2)) comparing laparoscopy vs. laparotomy in endometrial cancer [9]. Patients with clinical stage I–IIA uterine cancer were randomly assigned to laparoscopy (n = 1696) or open laparotomy (n = 920), including hysterectomy, salpingo-oophorectomy, pelvic cytology, and pelvic and para-aortic lymphadenectomy. Laparoscopy was initiated in 1,682 patients and completed without conversion in 1,248 patients (74.2 %). Conversion from laparoscopy to laparotomy was secondary to poor visibility in 14.6 %, metastatic cancer in 4.1 %, bleeding in 2.9 %, and other causes in 4.2 %. Laparoscopy had fewer moderate to severe postoperative adverse events than laparotomy (14 % v 21 %, respectively; P = .0001) but similar rates of intraoperative complications, despite having a significantly longer operative time (median, 204 v 130 min, respectively; P = .001). Hospitalization of more than 2 days was significantly lower in laparoscopy vs. laparotomy patients (52 % v 94 %, respectively; P = .0001). They concluded that laparoscopic surgical staging for uterine cancer is feasible and safe in terms of short-term outcomes and results in fewer complications and shorter hospital stay. Time to recurrence was the primary end point, with non-inferiority defined as a difference in recurrence rate of less than 5.3 % between the two groups at 3 years. The recurrence rate at 3 years was 10.24 % for patients in the laparotomy arm, compared with 11.39 % for patients in the laparoscopy arm, with an estimated difference between groups of 1.14 % (90 % lower bound, −1.278; 95 % upper bound, 3.996) [10]. Although this difference was lower than the pre-specified limit, the statistical requirements for non-inferiority were not met because of a lower-than-expected number of recurrences in both groups. The estimated 5-year overall survival was almost identical in both arms at 89.8 %. These results, combined with previous findings from this study of improved QOL and decreased complications associated with laparoscopy, are reassuring to patients and allow surgeons to reasonably suggest this method as a means to surgically treat and stage patients with presumed early-stage endometrial cancers.
Another prospective randomized trial is ongoing at Australian and the UK institutions, the Laparoscopic Approach to Cancer of the Endometrium (LACE) trial anticipated to randomize 590 patients to total laparoscopic hysterectomy and lymph nodal staging vs. standard, open surgery [11].
Disadvantages of laparoscopy:
Steep learning curve
Limited dexterity
Counterintuitive motion
Two-dimensional field
Limited depth perception
Ergonomic difficulty
Evidence for Robotic-Assisted Surgery
Obesity
Endometrial cancer is particularly suited for robotic surgery for several reasons. The majority of women with endometrial cancers are obese and at greater risk for postoperative wound complications and would benefit from a minimally invasive procedure with smaller incisions, resulting in less risk for wound problems. However, at the same time, obesity increases the degree of difficulty of management via laparoscopy, maybe to the extent that the level of difficulty may become prohibitive in accomplishing the operation. In a retrospective comparison of obese women and morbidly obese women undergoing traditional laparoscopic approach vs. robotic-assisted approach, better surgical outcomes were observed in the group undergoing robotic-assisted laparoscopy [12]. The group who underwent the procedure robotically had significantly shorter operating time, less blood loss, improved lymph node count, and shorter hospital stay suggesting that robotic-assisted laparoscopy greatly facilitates laparoscopic surgery in obese patients. In obese patients with greater abdominal surface area, adequate spacing between the ports and in turn clashing of the arms are seldom a problem.
Bernardini et al. [13] studied women with clinical stage I or II endometrial cancer and a BMI greater than 35 kg/m2 treated with robotic surgery at their institution between November 2008 and November 2010. These patients were compared with a historical cohort of similar patients who underwent laparotomy. A total of 86 women were analyzed in this study (robotic surgery, 45; laparotomy, 41). The overall intraoperative complication rate was 5.8 %. There was no statistical difference in age, number of comorbidities, BMI, prior abdominal surgery, and operative complications between the women who underwent robotic surgery vs. laparotomy. Postoperative complication rates were higher in the laparotomy group (44 % vs. 17.7 %; P = 0.007), and hospital length of stay was also higher in the laparotomy group (4 vs. 2 days; P = 0.001). There was no difference in rates of (pelvic) lymph node dissection; however, para-aortic node dissection was more common in the robotic surgery group.
Learning Curve
An analysis of robotic-assisted hysterectomy with lymphadenectomy vs. total laparoscopic hysterectomy with lymphadenectomy and laparotomy with total abdominal hysterectomy with lymphadenectomy was done by Lim PC et al. [14]. Data were categorized by chronologic order of cases into groups of 20 patients each. The learning curve of the surgical procedure was estimated by measuring operative time with respect to chronologic order of each patient who had undergone the respective procedure. Analysis of operative time for robotic-assisted hysterectomy with bilateral lymph node dissection with respect to chronologic order of each group of 20 cases demonstrated a decrease in operative time: 183.2 (69) min (95 % CI, 153.0–213.4) for cases 1–20, 152.7 (39.8) min (95 % CI, 135.3–170.1) for cases 21–40, and 148.8 (36.7) min (95 % CI, 130.8–166.8) for cases 41–56. For the groups with laparoscopic hysterectomy with lymphadenectomy and traditional total abdominal hysterectomy with lymphadenectomy, there was no difference in operative time with respect to chronologic group order of cases. It was concluded that the learning curve for robotic-assisted hysterectomy with lymph node dissection seems to be easier compared with that for laparoscopic hysterectomy with lymph node dissection for surgical management of endometrial cancer.
Survival Analysis
Retrospective study was conducted at two academic centers to compare the survival of women with endometrial cancer managed by robotic- and laparoscopic-assisted surgery [15]. A total of 183 women had robotic-assisted surgery and 232 women had laparoscopic-assisted surgery. With a median follow-up of 38 months (range 4–61 months) for the robotic and 58 months (range 4–118 months) for the traditional laparoscopic group, there were no significant differences in survival (3-year survival 93.3 and 93.6 %), DFS (3-year DFS 83.3 and 88.4 %), and tumor recurrence (14.8 and 12.1 %) for robotic and laparoscopic groups, respectively. Univariate and multivariate analysis showed that surgery is not an independent prognostic factor of survival. Robotic-assisted surgery yields equivalent oncological outcomes when compared to traditional laparoscopic surgery for endometrial adenocarcinoma.
A retrospective chart review was performed for all consecutive endometrial adenocarcinoma patients surgically staged with robotic-assisted laparoscopy at the University of North Carolina Hospital from 2005 to 2010 [16]. Demographic data, 5-year survival, and recurrence-free intervals were analyzed. Surgical staging was 85.2 % for stage IA, 80.2 % for stage IB, 69.8 % for stage II, and 69 % for stage III. Projected 5-year survival was 88.7 % for all patients included in the study. Nearly 82 % of cases were endometrioid adenocarcinoma, with papillary serous, clear cell, or mixed histology comprising 17.4 % of cases. Median follow-up time was 23 months, with a range of 0–80 months. Among stage IA, IB, II, and III patients, projected overall survival was 94.2 %, 85.9 %, 77.4 %, and 68.6 %, respectively. The results from this study demonstrate that robotic-assisted surgical staging for endometrial cancer does not adversely affect rates of recurrence or survival. These findings provide further evidence that robotic-assisted laparoscopic surgical staging is not associated with inferior results when compared to laparotomy or traditional laparoscopy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree