Minimally invasive surgical techniques are an emerging option in the staging and management of gastric cancer in the United States and elsewhere. Although much of the current knowledge about these approaches and their outcomes has been generated in Eastern countries, experience in the United States is growing. This article discusses both laparoscopic and robotic approaches to gastric cancer management. Important aspects of patient selection are emphasized. Surgical and oncologic outcomes are presented and compared with traditional open gastrectomy. Technical considerations are discussed along with comments on the learning curve to achieve proficiency in each approach.
Key points
- •
Minimally invasive approaches to the treatment of gastric cancer are emerging as a preferred option for well-selected patients.
- •
Appropriate patient selection is important.
- •
Minimally invasive techniques allow for decreased blood loss, less pain, and enhanced recovery.
- •
Laparoscopic and robotic approaches can provide equivalent oncologic outcomes when compared with open gastrectomy.
- •
Most data on minimally invasive gastrectomy come from Eastern countries, but the Western experience is growing.
Introduction
Although gastric cancer is less common in the United States than in other areas of the world, it remains an important contributor to cancer death and is associated with worse survival than in Eastern countries. In 2012, there were an estimated 951,600 new diagnoses of gastric cancer and 723,100 deaths due to gastric cancer worldwide. The overall incidence of gastric cancer in the United States is increasing from 22,000 to 25,000 new cases per year, with a particular increase in incidence of gastroesophageal junction and gastric cardia tumors. Additionally, in the young population of 25 to 39 year olds, the United States has seen a 70% increase in noncardia gastric cancer over the past several years. Moreover, gastric cancer may manifest in a variety of histologic, anatomic, and genetic patterns, which influences the surgical approach and requires a customized and multimodality treatment plan for each patient. Gastrectomy with curative intent remains the only treatment that can offer potential for cure in gastric cancer patients. Over the past 20 years, minimally invasive techniques have emerged that enhance the surgical armamentarium of approaches to both complete gastric cancer staging and curative resection. Multiple randomized trials comparing laparoscopic to open gastrectomy have proved oncologic equivalency of the 2 approaches and have demonstrated favorable outcomes in postoperative recovery with minimally invasive approaches. As a result, minimally invasive surgery is emerging as a preferred option in the treatment of well-selected gastric cancer patients. As knowledge grows regarding the conduct and outcomes of robotic-assisted approaches, this new technique is being adopted both in the United States and elsewhere, with favorable outcomes in retrospective series. This article discusses the emerging role of both laparoscopic and robot-assisted approaches to gastric cancer management.
Introduction
Although gastric cancer is less common in the United States than in other areas of the world, it remains an important contributor to cancer death and is associated with worse survival than in Eastern countries. In 2012, there were an estimated 951,600 new diagnoses of gastric cancer and 723,100 deaths due to gastric cancer worldwide. The overall incidence of gastric cancer in the United States is increasing from 22,000 to 25,000 new cases per year, with a particular increase in incidence of gastroesophageal junction and gastric cardia tumors. Additionally, in the young population of 25 to 39 year olds, the United States has seen a 70% increase in noncardia gastric cancer over the past several years. Moreover, gastric cancer may manifest in a variety of histologic, anatomic, and genetic patterns, which influences the surgical approach and requires a customized and multimodality treatment plan for each patient. Gastrectomy with curative intent remains the only treatment that can offer potential for cure in gastric cancer patients. Over the past 20 years, minimally invasive techniques have emerged that enhance the surgical armamentarium of approaches to both complete gastric cancer staging and curative resection. Multiple randomized trials comparing laparoscopic to open gastrectomy have proved oncologic equivalency of the 2 approaches and have demonstrated favorable outcomes in postoperative recovery with minimally invasive approaches. As a result, minimally invasive surgery is emerging as a preferred option in the treatment of well-selected gastric cancer patients. As knowledge grows regarding the conduct and outcomes of robotic-assisted approaches, this new technique is being adopted both in the United States and elsewhere, with favorable outcomes in retrospective series. This article discusses the emerging role of both laparoscopic and robot-assisted approaches to gastric cancer management.
The role of diagnostic laparoscopy and peritoneal washing cytology
Laparoscopy has emerged as an important staging modality for locally advanced gastric cancer. Patients who are found to have T3 or greater disease or node-positive disease, by CT scan and/or endoscopic ultrasonography (EUS), benefit from staging laparoscopy because the findings may alter the management strategy and treatment intent in a significant proportion of patients.
In a study of 657 patients at Memorial Sloan Kettering Cancer Center (MSKCC), diagnostic laparoscopy was performed for patients with gastric adenocarcinoma and staging CT scans showing no definitive evidence of metastatic disease. In the entire study population, visible peritoneal metastases were identified in 31% of patients, suggesting a high incidence of radiographically occult metastatic disease. Clinicopathologic predictors of identifying radiographically occult peritoneal metastases were location of the tumor at the gastroesophageal junction or involving the entire stomach, poor differentiation on histology, and age less than or equal to 70 years. Imaging predictors of identifying peritoneal metastases at laparoscopy were lymphadenopathy greater than 1 cm and T3/T4 tumors. EUS may also be used to stratify risk for radiographically occult metastatic disease. EUS findings in 94 patients were correlated with their diagnostic laparoscopy results in a retrospective study that identified patients as high risk when EUS showed T3, T4, and/or N1 disease; all others were considered low risk. The high-risk group had a 25% likelihood of peritoneal metastatic disease compared with 4% in the low-risk group. Because peritoneal metastatic disease changes treatment intent from curative to palliative, diagnostic laparoscopy, therefore, can alter the goals of therapy in a large proportion of patients considered to have locally advanced disease at presentation.
Diagnostic laparoscopy additionally provides the opportunity to collect peritoneal washings for cytology, in the absence of visible peritoneal disease. Several studies have demonstrated that the presence of cancer cells in peritoneal washings of gastric cancer patients is a significant predictor of mortality from the disease. In a study of 1297 patients with gastric cancer who underwent peritoneal lavage, the population with positive cytology had a 5-year survival rate of only 2%. At MSKCC, in a study of 371 patients who underwent R0 resection for gastric cancer, those who had positive peritoneal cytology had a significantly reduced median survival of 14.8 months compared with 98.5 months in those with negative cytology. In multivariate analysis, positive cytology was the strongest predictor of death from gastric cancer. Other studies have also convincingly demonstrated reduced survival in those with positive peritoneal cytology. Owing to this evidence, positive peritoneal cytology is considered metastatic (M1) disease according to the American Joint Committee on Cancer staging system. As such, laparoscopy plays a vital role in the staging of gastric cancer patients.
Selective use of laparoscopic approaches
Appropriate selection of patients and optimal technical approach are paramount for good outcomes and require deep understanding of gastric cancer and the underlying differences in biological behavior and natural history of diffuse and intestinal types of the disease. Thin patients with few comorbidities and early cancers are the most ideal patients for a laparoscopic approach. As the learning curve progresses and the surgeon’s understanding of the biological behavior of the disease matures, indications may be broadened.
As with any novel surgical approach and technique, a relative contraindication includes technical expertise of the surgeon and experience. It is crucial to keep in mind that the primary goal is to provide an oncologically equivalent operation via the minimally invasive approach. For a surgeon who is early in his or her learning curve, relative contraindications include patients with a high body mass index and more advanced tumors that require possible en bloc resection of associated invading organs and structures. Patients with comorbidities, including severe pulmonary or cardiac disease, may benefit from a shorter anesthetic time and thus an open approach. Lastly, patients with multiple previous abdominal operations or extensive adhesions as well as those who have completed neoadjuvant therapy may have more technically complicated cases and should therefore be selected judiciously.
Outcomes of laparoscopic gastrectomy
A large volume of research regarding outcomes for the laparoscopic approach compared with the open approach has been published since the first report by Kitano and colleagues in 1994. Given the increased incidence of early gastric cancer in East Asia, the Eastern experience with laparoscopic gastrectomy has been more robust than in the United States, where laparoscopic approaches for gastric cancer have been more slowly accepted. Given different results comparing outcomes of gastric cancer surgery in the East and the West as well as differing epidemiology of cases, Eastern-derived data should be extrapolated cautiously with regard to Western patients. Table 1 lists the major findings of several recent randomized trials of laparoscopic compared with open gastrectomy.
| Study, Year | Eligibility | Procedure | Lymph Node Dissection Extent | Number of Patients | Operative Time Less | Blood Loss Less | Number of Lymph Nodes Retrieved | Hospital Stay | Morbidity | Mortality |
|---|---|---|---|---|---|---|---|---|---|---|
| Kitano et al, 2002 | cT1 | LADG/ODG | NR | 14/14 | ODG | LADG | Equivalent | Equivalent | Equivalent | Equivalent |
| Fujii et al, 2003 | cT1 | LADG/ODG | NR | 10/10 | ODG | Equivalent | NR | NR | Equivalent | Equivalent |
| Hayashi et al, 2005 | cT1 | LADG/ODG | NR | 14/14 | ODG | Equivalent | Equivalent | LADG | NR | Equivalent |
| Huscher et al, 2005 | cT1-4 N0-2 | TLGD/ODG | D1, D2 | 30/29 | Equivalent | TLGD | Equivalent | TLGD | Equivalent | Equivalent |
| Lee et al, 2005 | cT1 | LADG/ODG | D2 | 24/23 | ODG | Equivalent | Equivalent | Equivalent | LADG | NR |
| Kim et al, 2008 | cT1 N0-1 | LADG/ODG | D1, D2 | 82/82 | ODG | LADG | ODG | LADG | NR | NR |
| Kim et al, 2010 | cT1-2N0-1 | LADG/ODG | D1, D2 | 179/163 | ODG | LADG | NR | NR | Equivalent | Equivalent |
| Cai et al, 2011 | cT2-3 | LAG/OG | D2 | 49/47 | OG | Equivalent | Equivalent | Equivalent | Equivalent | NR |
| Sakuramoto et al, 2013 | cT1 | LADG/ODG | D1 | 31/32 | ODG | LADG | Equivalent | Equivalent | Equivalent | NR |
| Takiguchi et al, 2013 | cTNMI | LADG/ODG | D1, D2 | 20/20 | ODG | LADG | Equivalent | LADG | NR | NR |
| Aoyama et al, 2014 | cTNMI | LADG/ODG | D1, D2 | 13/13 | ODG | LADG | Equivalent | NR | Equivalent | Equivalent |
Perioperative Factors
In a recent meta-analysis of 7 randomized controlled trials totaling 390 patients comparing laparoscopic versus open distal gastrectomies, the laparoscopic approach was found to have longer operative time but was also associated with less blood loss, fewer analgesics administered, faster recovery, and shorter postoperative hospital stay. Similar findings have been reported by Viñuela and colleagues looking at various factors comparing the laparoscopic and open approaches. Specifically, operative time was found on average 48 minutes longer via the laparoscopic approach compared with the open procedure. Some studies suggest this difference is related to learning curve issues. This study also found operative blood loss lower for the laparoscopic approach.
Multiple other retrospective series as well as randomized controlled trials have demonstrated less postoperative pain and postoperative ileus, earlier oral intake and ambulation, and better postoperative respiratory function in favor of the laparoscopic group.
Length of stay has been reported lower in many Western series; however, this finding is more likely correlated to differences in practice patterns comparing Western and Eastern countries. In the recent meta-analysis, discussed previously, the investigators analyzed this outcome and found that hospital stay was significantly reduced by more than 3 days after laparoscopic distal gastrectomy compared with the conventional open procedure.
Postoperative Morbidity and Mortality
The 30-day postoperative complication rates of laparoscopic gastrectomy have been reported to range between 10% and 20% in Eastern country studies whereas studies from Western countries have demonstrated a slightly higher morbidity rate (20% to 30%). Possible explanations include an older cohort of patients with increased comorbidities in Western countries. In a recent updated meta-analysis of 14 randomized controlled trials, which compared safety and efficacy of laparoscopic gastrectomy with open gastrectomy for resectable gastric cancer totaling 2307 patients (1163 in laparoscopic and 1144 in open), laparoscopic gastrectomy was associated with decreased blood loss, length of hospital stay, and overall postoperative morbidity and improved postsurgical recovery. In addition, the laparoscopic group demonstrated accelerated time to first flatus, first walking, and oral intake as well as reduced frequency of analgesic administration. No significant difference in number of retrieved lymph nodes, mortality, recurrence, or long-term overall survival and disease-free survival was observed.
Perioperative Complications
Unexpected intraoperative events are usually the major reason for conversion from a laparoscopic to an open procedure. Most of these are associated by direct handling of organs with retractors and dissection devices. To prevent a catastrophic outcome, it is crucial that the surgical team is aware of these potential issues and prepared for immediate conversion to an open procedure without any delays if such an incident occurs. Factors, such as higher body mass index and more extensive lymphadenectomies, have been described as associated with a higher risk of intraoperative events.
These complications are in general rare. Most conversions to an open procedure are related to bleeding events. One retrospective study from Korea calculated a 2.6% rate of intraoperative complications; 56% were bleeding events, all requiring conversion to open surgery. In the meta-analysis by Viñuela and colleagues, 63% of the conversions to open gastrectomy were due to intraoperative bleeding. The right gastroepiploic vessels during the dissection of the infrapyloric area and common hepatic and splenic arteries during lymphadenectomy are the most common sources of bleeding reported. Another reason for conversion is suspected injuries to the pancreas while performing lymph node dissection or lacerations of the distal esophagus during laparoscopic total gastrectomy.
Approximately 1% of patients experience postoperative hemorrhage after laparoscopic gastrectomy. Most of the time, bleeding from staple or suture lines can be managed nonoperatively. For any laparoscopic procedure, bleeding from the port sites must be considered, in particular the specimen extraction site. Postoperative pseudoaneurysms are rare events in both open and laparoscopic approaches; however, they can be sources of major bleeding and sometimes only confirmed by angiography. In such cases, the treatment of choice is selective embolization in general but the surgeon should also have a low threshold for re-exploration if clinically indicated.
Wound complications in the laparoscopic setting are uncommon and occur in approximately 2% of patients. The extraction site is the wound most frequently affected by an infection. As with any surgical procedure, administration of appropriate preoperative antibiotics is recommended. To decrease further potential contamination, preventive measures can be used, including using an extraction bag for the specimen and wound protective sheaths.
Across the literature, incisional hernia rates have been reported less frequent for patients undergoing a laparoscopic approach compared with open techniques. In a systemic review and meta-analysis, Kössler-Ebs and colleagues evaluated 24 randomized controlled trials with 3490 patients comparing incisional hernia rates after laparoscopic versus open abdominal surgery. In their analysis, incisional hernias were significantly reduced in the laparoscopic group (4.3%) compared with the open group (10.1%, P = .0002). Moreover, laparoscopically assisted procedures did not show a significant reduction of incisional hernias compared with open surgery.
In the authors’ experience, adequate technique of fascial closure is the single most important preventive measure to avoid port-site hernias. Closure of the larger port sites (>12 mm) using a fascia closure device is recommended.
Long-term Complications and Outcomes
As discussed previously, the safety of laparoscopic gastrectomy has been well established in the literature. Okabe and colleagues most recently evaluated various surgical techniques for laparoscopic esophagojejunostomy and established that there was no superiority of any particular method (circular or linear staplers). The incidence of anastomosis-related complications varied among studies and the overall complication rate of laparoscopic gastrectomy was similar to that of the open procedure.
Similar findings have been described regarding the effectiveness and oncologic results of the laparoscopic technique. In a recent review by Son and colleagues, cumulative results from multiple trials did not show significant differences in terms of survival rate or recurrence after surgery based on long-term follow-up evaluation. Although multiple trials and review articles have shown that the number of lymph nodes retrieved were equivalent in the 2 groups, some studies have demonstrated a higher lymph node count. The clinical significance of that difference remains unclear, however, given the same oncologic and survival outcomes.
As reported by the authors’ recent meta-analysis, postoperative mortality after laparoscopic gastrectomy is less than 1% and comparable after the open technique. This has been confirmed by various other studies. Moreover, the authors demonstrated in a meta-analysis that the odds of developing serious complications after laparoscopic gastrectomy were observed similar to that of the open procedure. Major features of the surgical procedure that encompass the main source of possible postoperative morbidity (extent of gastric resection, lymphadenectomy, and reconstruction) have all been described as similar in both techniques.
One long-term complication that has been reported as slightly different is potential for reduced incidence of adhesive ileus after a laparoscopic approach. This finding has been attributed to the less inflammatory and immunologic peritoneal reaction induced by laparoscopic surgery. A recent systematic study of more than 400,000 patients followed after abdominal surgery demonstrated an incidence of bowel obstruction for cholecystectomy, gynecologic procedures, and colorectal surgery. These results can likely be extrapolated to other laparoscopically performed abdominal procedures, including gastrectomies. A reduced incidence of obstructive adhesions associated with laparoscopic gastrectomy specifically has also been reported in a few smaller comparative studies, although the evidence is still somewhat controversial.
Quality of life
Minimally invasive techniques have been applied with the goal of enhanced recovery and improved quality of life for patients. True quality of life is challenging to measure and report and is influenced by many factors, both objective and subjective. The quality of life after gastrectomy may further be influenced by the extent of resection, incidence of complications, or disease recurrence, which may not vary with the approach used. There is, however, evidence of improvements in quality of life when minimally invasive approaches are applied to selected patients. A randomized controlled trial performed in South Korea assessed quality of life in patients with early gastric cancer randomized to either open or laparoscopic distal gastrectomy. Validated questionnaires were administered up to 3 months after surgery. In the laparoscopic arm, quality-of-life scores were better in multiple domains, including physical, emotional, social, and global. Pain, sleep, appetite, diet, and body image were also improved with laparoscopic resection compared with open gastrectomy.
Technical considerations for laparoscopic gastrectomy
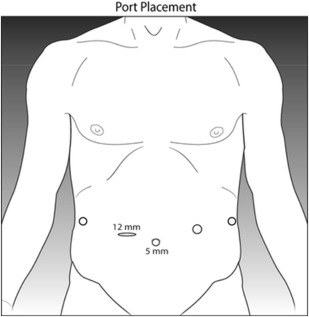
The technique of laparoscopic gastrectomy has been well described. The patient is placed supine on the operating table. Pneumoperitoneum can be established in the standard fashion, either by Veress needle or Hasson technique. In general, 5 ports are used, with a 12-mm trocar in the right upper quadrant 4 additional 5-mm trocars as shown in Fig. 1 . The patient should be placed in steep reverse Trendelenburg position, which may be safely done with the use of a footboard on the bed. Energy sealing devices may be used to facilitate the dissection. Linear staplers introduced via the 12-mm port may be used to divide the bowel, stomach, or esophagus, as dictated by the anatomic location of the tumor.
Reconstruction of gastrointestinal continuity may be established using a variety of techniques. These include both intracorporeal hand-sewn anastomoses, which may be technically challenging, and stapler devices. In particular, an esophagojejunostomy may be created either with linear staplers or by using a transoral anvil device and circular stapler. Gastrojejunostomy and jejunojejunostomy, if Roux-en-Y reconstruction is chosen, are readily performed with a linear dividing stapler. Intracorporeal hand-sewn closure of the anastomotic gap left by the stapler is typically performed.
Learning curve for laparoscopic gastrectomy
Many factors influence the learning curve for laparoscopic gastrectomy. The technical challenges of laparoscopic gastrectomy, although real, are only 1 consideration. Surgeon training, experience, and volume may affect the time to proficiency, and hospital support for and experience with laparoscopic surgery programs may also influence the learning curve. Additionally, the different types of gastric resection, ranging from distal gastrectomy to total gastrectomy, pose a range of technical challenges. For example, a stapled gastrojejunostomy is less technically demanding than esophagojejunostomy.
Few publications address the learning curve for laparoscopic gastrectomy in a systematic way. Furthermore, most of the literature has been generated in Eastern countries, where the incidence of gastric cancer is greater than in the United States. One study reported the outcomes of the first series of laparoscopic distal gastrectomies performed by a single surgeon in South Korea who previously had extensive experience with open gastrectomy ; 177 patients with early gastric cancer undergoing distal gastrectomy were included in the study, with 102 in the laparoscopic group and 71 in the open group. The laparoscopic cases were divided into early (n = 50) and late (n = 52) groups. In the late group, mean operative time was shorter than in the early group (190 vs 230 minutes) and mean lymph node retrieval was greater (30 vs 45 nodes). The open group had the fastest mean operative time (154 minutes) and the greatest mean lymph node retrieval of 38 nodes.
Another series of 100 patients who underwent laparoscopic gastrectomy was divided into 5 groups of 20 patients according to the surgeon’s level of experience. For surgeons with laparoscopic experience of more than 60 cases, there were no conversions to open and the operative time approached that of a comparison group of 67 open gastrectomies (227 vs 232 minutes). Hospital length of stay was also shorter for surgeons with experience of at least 60 cases. Blood loss was decreased after an experience of 20 cases.
Similar studies also suggest a learning curve to proficiency of approximately 50 to 60 laparoscopic gastrectomies. These studies, however, included only early gastric cancers in selected patients. The learning curve may be more difficult to overcome in unselected patients with advanced disease, in particular those requiring extended lymphadenectomy. Surgeons should be aware of their level of proficiency when selecting patients for minimally invasive approaches. The learning curve for robotic-assisted gastrectomy may require fewer cases to achieve proficiency (discussed later).
Robotic-assisted gastrectomy
Robotic-assisted gastrectomy is the newest approach to curative-intent gastric surgery. Use of the robotic platform for gastric cancer treatment was first described in 2003, with the first report from the United States appearing in 2007. In addition to the advantages of general minimally invasive techniques, including reduced hospital lengths of stay and postoperative discomfort, the robotic approach has technical advantages over traditional laparoscopic surgery. The robotic camera provides 3-D visualization and magnification that aid in fine dissection during gastrectomy. Articulating instruments provide much-improved dexterity and finer control, which is advantageous for precision.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree