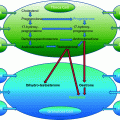
Fig. 19.1
INVO principle. INVOcell device is permeable to CO2 gas and allows the equilibrium between the pCO2 of the vaginal/rectal venous plexus (~5–6%) and the pCO2 of the culture medium (0.04–0.05%). Also, the INVOcell device is permeable to O2 gas and allows the equilibrium between the pO2 of the culture medium (20%—pO2 of air) and the pO2 of the vaginal/rectal venous plexus (~5%)
Infertile couples during conventional assisted reproduction treatments undergo an intense process that involves psychological and economical implications, in addition to the medical procedures that represent risks and secondary effects [18, 19]. A significant aspect of the INVO procedure lies in the psychological benefit that is created among the patients who feel closely involved in the process of fertilization and embryo development, which generates a high level of acceptance of the procedure and giving a more natural approach of the assisted conception.
INVOcellTM Device Components
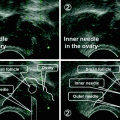
The INVOcellTM device is a 2-inch plastic capsule and comprises an inner chamber, an outer rigid shell, and a retention system. The parts of the INVOcellTM are best shown in (Fig. 19.2). The inner chamber and the outer rigid shell are made from biocompatible plastic material (polypropylene) which is permeable to CO2 gas and O2 gas. The polypropylene material enable CO2 gas and O2 gas permeate the inner chamber wall from the vagina into contained culture medium. The INVOcellTM device is ISO-10993 tested to assess toxicity, biocompatibility, comfort, and retention within the vaginal cavity [2]. Description of each INVOcellTM part is as follows:
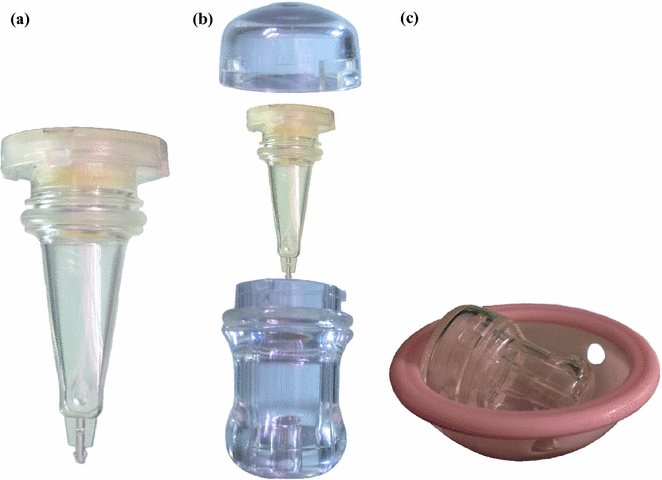

Fig. 19.2
Component parts of the INVOcell device. a Inner chamber. b Outer rigid shell. c Assembled INVOcell device with its retention system
Inner Chamber
Contains the culture medium and the gametes;
The volume of the chamber is 1.1 ml;
At the bottom of the main chamber, a microchamber collects the embryos;
At the top of the chamber, a rotating valve allows several openings and closings without the introduction of air or contamination of the culture medium; and
The rotating valve has a small orifice at a bottom of small well which prevents variations of pH of the culture medium and loss of gametes due to possible overflow while filling.
Outer Rigid Shell
Protects the inner chamber from vaginal contaminations;
Keeps the inner chamber in a sterile environment;
It has a smooth external surface to prevent any lesions or irritations of the vaginal cavity or cervix; and
It has a locking position to prevent any unexpected device opening during vaginal incubation.
Retention System
The diaphragm has a perforated membrane that avoids accumulation of vaginal secretions and preventing vaginal infections during intravaginal culture incubation and
Avoid the INVOcellTM device expulsion from the vaginal cavity.
INVO Procedures
INVO procedure has contributed to a returned interest in natural and more physiological IVF protocols that produce less embryos and are safer for the female. Using INVOcellTM allows maturation of gametes, fertilization, and embryo development into the best natural incubator, the vaginal cavity. This alternative option for conventional IVF treatment is considered as a simplified replacement of the IVF complex laboratories. This has reduced the necessity of sophisticated laboratory instrumentation simplifying the process of IVF and embryo development. Natural cycle and mild stimulation protocols with the INVO procedure also have contributed to the simplicity, low complication rates, and low cost of the INVO cycle.
Modified Natural Cycle
The monitoring of natural cycle is simple and inexpensive. Our modified natural protocols begin at the first day of the menstrual cycle evaluating the ovarian reserve by a pelvic ultrasound examination. On day 7th of menstrual cycle, a pelvic ultrasound is made to evaluate follicular size and also to identify a possible dominant follicle. At that moment, we start clomiphene citrate (CC) at doses of 25 mg per day (good ovarian reserve) or 50 mg per day (low ovarian reserve) at 9 am for 5 days to reinforce the follicular recruitment. At 13th day of the menstrual cycle, we start indomethacin (50 mg every 8 h per day, as used in mild protocols and discussed later) if the leading follicle reached 15 mm of diameter and is used to prevent premature ovulation. Triggering of ovulation is performed by GnRH-a (injection of 1 mg of leuprolide acetate) when the size of the follicle reaches 17 mm and the blood estradiol levels are around 250 pg/ml. Oocyte retrieval is performed 36 h after ovulation trigger and continues with the INVO laboratory protocol.
Mild Ovarian Stimulation and INVO: Clinical Experience
Seven years ago, the conventional protocols using high doses of gonadotropins have been discontinued in our clinic. At present, we have designed a new era in assisted reproductive (AR) techniques. Our main goal is to offer more physiological, natural, and safe treatments. This new alternative in controlled ovarian stimulation (COS) has been achieved by using low and mild doses of hormonal medications that support affordable treatments with the purpose of reaching a significant number of infertile couples in our population in terms of cost–benefit. Mild IVF and INVO procedure offer an advantage simplifying the laboratory equipment and manipulations needed, which might decrease the costs and allow a widespread application for patients who cannot afford traditional IVF [20] as it would be the case in developing countries, where the access to cost-effective infertility treatment is limited. We have focused on the following important factors for protocol preparation:
Increased rates of infertility cases in our population.
Physical, psychological, and emotional effects derived from conventional COS.
Multiple risks related to multiple pregnancies.
Focusing on single embryo transfer.
Availability of clinically used medications such as non-selective inhibitors of cyclooxygenase type 2 (COX-2) as an economical method to achieve blocking of ovulation.
Technological advances on different fields of clinical science: endocrinology, ultrasonography, and embryology.
The cost of conventional COS treatments.
The current and progressive increase in demand for assisted reproductive treatments.
The commitment to provide affordable fertility treatments to the public health service in our population.
The convenience of these fertility treatments to the middle-class population.
Different purposed methods of mild COS have been designed based on the global scientific evidence and joined to adjuvant therapies as hyperbaric oxygen sessions and preconceptional preparation cycle that enhances the effectiveness of AR treatments.
Hyperbaric Oxygen Therapy (HBOT)
HBOT consists of the medical use of oxygen at high doses (oxygen saturation close to 100%), during short periods of time (generally one hour), and at level higher than atmospheric pressure (oxygen pressure comparable to the sea level). The patient breathes pure oxygen in a pressured chamber that induces positive effects to the uterus such as:
Stimulation of endometrial and follicular angiogenesis.
Subendometrial, endometrial, and follicular hyperoxia.
Stimulation of the endometrial pinopode formations.
Increases sensibility of estrogenic receptors and hormonal mediators that modulate uterine immune response.
Improves endometrial receptivity.
Improves embryo implantation rates (Table 19.1).
Table 19.1
Results from treated and non-treated patients under hyperbaric oxygen (HBO) sessions during 2009–2010 at the fertility and sterility Colombian centre (CECOLFES)
HBO patients (n = 305)
Non-HBO patients (n = 80)
MII
92%
74.3%
Fertilization
80%
90%
48 h
76%
60%
72 h
72%
54%
Clinical pregnancies
129 (42.3%)
22 (28%)
Biochemical pregnancies
5
2
Miscarriages
10
4
Avg. age
26–48 años
23–50 años
Non-tolerance
79 (25%)
Claustrophobia
l
Ear pain
78
Preconceptional Preparation
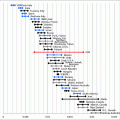
As a part of COS treatments, we have suggested strategies of previous preparation using birth control pills. Those pills should be started at the first day of the menstrual cycle throughout 21 days. Once the patient takes the 21st pill of preparation, we start the administration of estradiol valerate 2 mg (tablets) for three continuous days. The bleeding will occur on a scheduled basis on the 26th and 29th days of the menstrual cycle. The main goal is to harmonize the FSH and LH levels, the follicular development, and the oocyte–endometrial quality. Also, preconceptional preparation allows a regular endometrial desquamation reducing the possibilities of ovarian cyst formation that impedes the beginning of COS (Fig. 19.3).
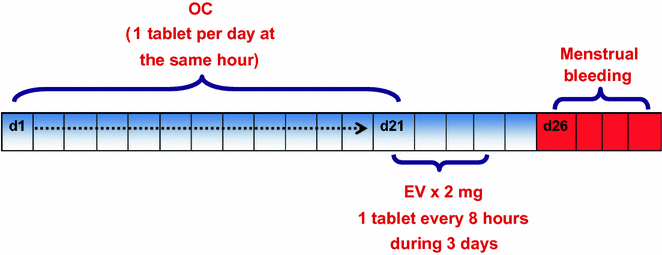

Fig. 19.3
Preparation preconceptional protocol fertility and sterility Colombian Center (CECOLFES) (OC Oral contraceptives, EV Estradiol valerate)
Mild Ovarian Stimulation Protocols Used on INVO Procedure
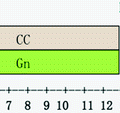
We have established different protocols based on the concentration of medications used for ovarian stimulation: clomiphene citrate (CC) and human menopausal gonadotropin (hMG) and the total days of treatment (Fig. 19.4).
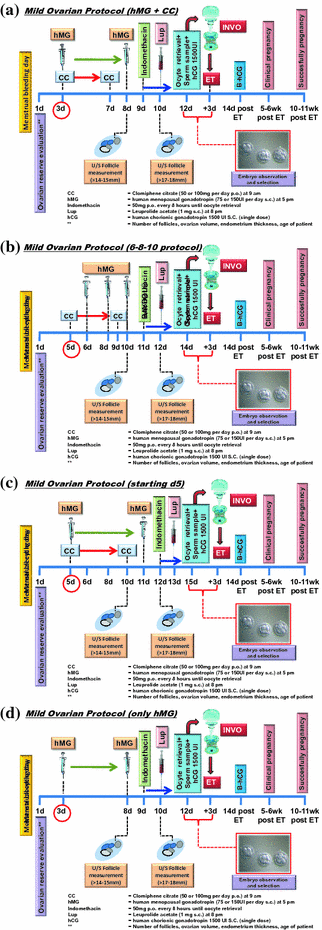

Fig. 19.4
a–d Mild ovarian stimulation protocols used on INVO procedure. Further information read the text
Protocol I
hMG, 75 IU, for 5 continuous days, starting first dose at the 3rd day of the menstrual cycle
Protocol II
hMG, 150 IU, for 6 continuous days, starting first dose at the 3rd day of the menstrual cycle
Protocol III
Combo I: CC 50 mg + hMG, 75 IU, for 6 continuous days
Combo II: CC 100 mg + hMG, 75 IU, for 6 continuous days
Combo III: CC 50 mg + hMG, 150 IU, for 6 continuous days
Combo IV: CC 100 mg + hMG, 150 IU, for 6 continuous days
Protocol IV
CC 50 or 100 mg, +75 or 150 IU, at the 6th, 8th, and 10th days of the menstrual cycle
Protocol V
CC 50 or 100 mg, for 5 continuous days, starting first dose at the 3rd day or the 5th day of the menstrual cycle.
Blocking of Follicular Rupture
On IVF mild protocols, we have established the use of nonsteroidal anti-inflammatory (NSAID) medicines derived from the indol-3-acetic acid. These medicines, such as indomethacin or acemetacin, are non-selective inhibitors of the production of potent inflammatory mediators derived from COX-2 enzimatic action. COX-2 molecule is essential for the biosynthesis of prostaglandins E2 (PGE2) and F2 (PGF2). Prostaglandins are molecules derived from fatty acids stored in the cell membrane, and its biosynthesis can be divided into three phases: 1) releasing of arachidonic acid from the membrane phospholipids by the phospholipase enzyme action, 2) conversion of the arachidonic acid into an unstable intermediate prostaglandin called PGH2, by the action of COX-2, and 3) conversion of the PGH2 molecule in different specific active prostanoids for each cell [21]. Acemetacin and indomethacin have inhibitory action over COX-2 molecule. Prostaglandins are critical mediators of many biological procedures involved in female fertility such as ovulation, luteolysis, embryo implantation, and delivery [21]. The close relationship between LH surge and PGE2 and PGF2 concentrations in the ovarian follicles, just previous to the ovulation, suggests that the granulose cells might produce those two molecules. Acemetacin and indomethacin have a direct effect on the ovary inhibiting the preovulatory concentration of PGE2 and PGF2 into the follicle giving raise to a lag of oocyte releasing. Also, all molecular events involved in the follicular rupture including increased vascular permeability, collagenolysis, and local inflammatory events are inhibited.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree