10 Minimally Invasive Breast Biopsy
Introduction
Manufacturers of ultrasound equipment have realized the importance of breast-specific hardware and software. High-frequency, broadband width transducers (up to 17 MHz) have allowed for exquisite resolution of very small structures within the breast and the detection of subcentimeter cancer as well as ductal carcinoma in situ (DCIS) (Fig. 10-1). Coded harmonics and spatial compound imaging techniques reduce artifacts and allow for more confident lesion visualization. Color Doppler flow can be used in the setting of complex cysts and intraductal lesions to detect blood flow (Fig. 10-2). All these improvements have brought breast ultrasound to a level which makes it an indispensable tool in breast diagnosis.
Stereotactic mammographic units have digital imaging, which provides superior contrast resolution and near instantaneous image display compared with film screen imaging (Fig. 10-3). This has improved both efficiency and accuracy of the biopsy procedures. In addition, many manufacturers now offer digital mammographic spot-imaging capabilities. There are also units designed exclusively for specimen radiography (Fig. 10-4). These devices allow for immediate evaluation of breast tissue for the presence of calcifications and post-biopsy metallic markers, thus speeding the performance of both stereotactic biopsies as well as surgical lumpectomies. Finally, several manufacturers now have full-field digital mammography, which provides superior contrast resolution, increased throughput, and reduced radiation exposure compared with standard film screening mammography (Fig. 10-5). Full-field digital has also been shown to be superior to film screen for women with dense breasts, premenopausal women, and women under 50 years old.1
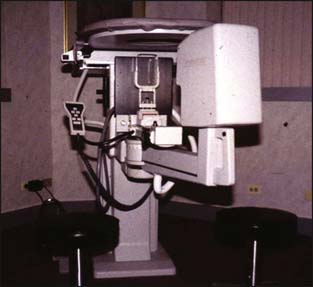
Figure 10-3 Mammo Test Stereotactic Biopsy Table.
(Courtesy of Fischer Imaging, Inc. Denver, Colorado.)
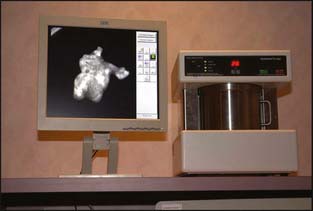
Figure 10-4 DX 50 Core Specimen Radiography System.
(Courtesy of Faxitron X-Ray, Lincolnshire, Illinois.)

Figure 10-5 Selenia Digital Mammography.
(Courtesy of Lorad, a Hologic Company, Bedford, Massachusetts.)
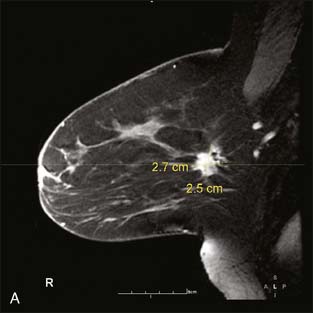
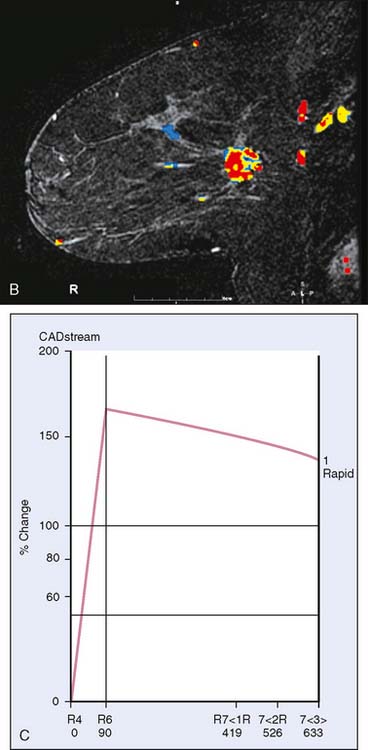
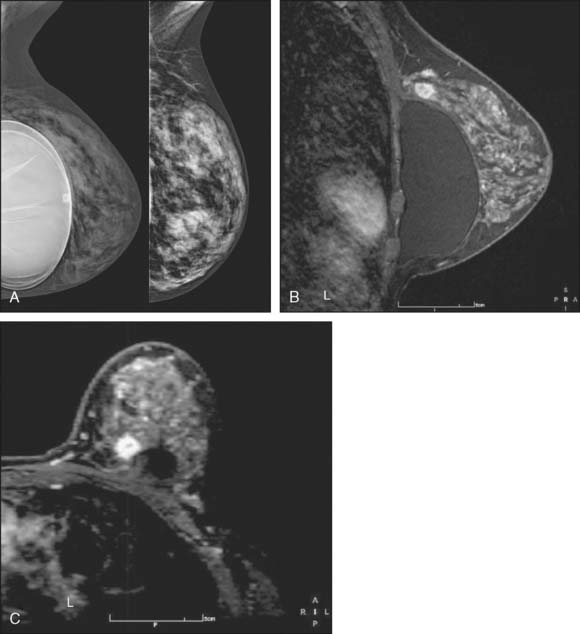
Breast MRI, though expensive, is an exceptionally helpful tool in treatment planning. There are in general two types of breast MRI, both of which use gadolinium contrast injection: dynamic breast MRI and high-resolution breast MRI (Fig. 10-6A through C). Dynamic breast MRI images the breast multiple times in the first few minutes after contrast injection. This allows for analysis of contrast uptake and washout with an improved specificity compared with the high-resolution breast MRI. High-resolution breast MRI results in very high sensitivity with impressive spatial resolution. The specificity is not as high as with dynamic imaging, however.2,3 Most modern breast MRI equipment and software can do both dynamic and high-resolution imaging while imaging both breasts simultaneously.
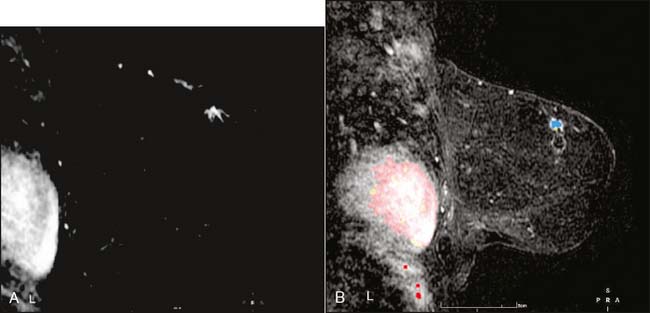
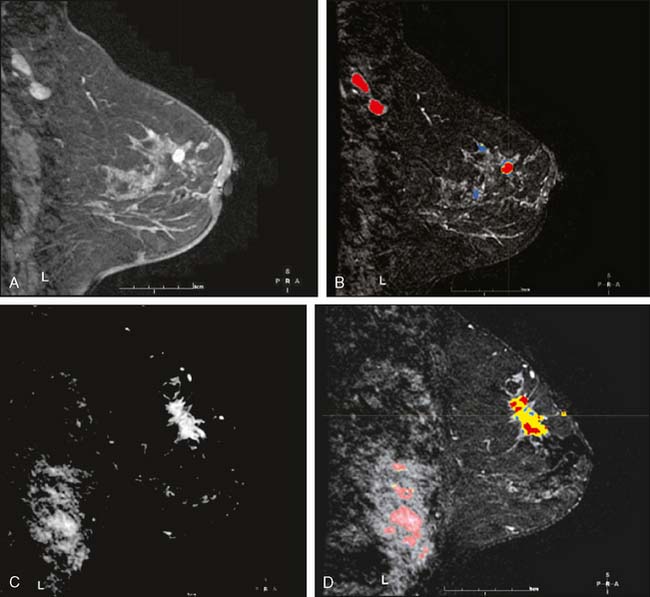
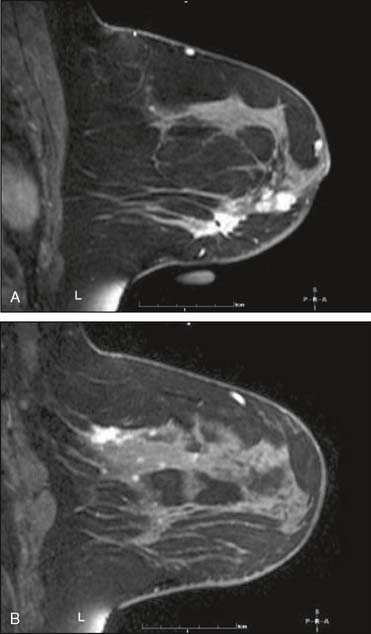
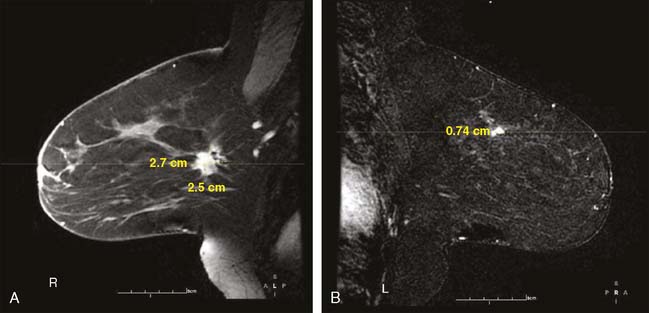
Our experience has been that the increased specificity associated with dynamic imaging is not sufficient to eliminate the need for biopsy in enhancing lesions (Fig. 10-7A and B). However, in the patient with multiple enhancing nodules with benign morphology or diffuse fibroglandular enhancement, the dynamic information may assist in identifying the more suspicious lesion(s) to target. We rely heavily on the morphologic characteristics gleaned from the high-resolution images (Fig. 10-8A through D). We have found that MRI is most useful in the preoperative assessment of a patient with a biopsy-proven breast carcinoma.4–7 In these instances, the true extent of disease is much better appreciated than with standard mammographic images, and the surgery can be appropri ately tailored. In addition, in approximately 5% to 7% of patients, otherwise unsuspected carcinoma is detected in a different quadrant of the same breast (multicentric disease) (Fig. 10-9A and B). Also, in approximately 5% to 7% of patients, otherwise unsuspected carcinoma is found in the opposite breast8–10 (Fig. 10-10A and B). More frequently, we are performing breast MRI on high-risk patients. These include patients with BRAC 1 and BRAC 2 genetic risk and patients with previous high-risk biopsy results or previous breast cancer11–16 (Fig 10-11A through C). Occasionally, MRI is used for monitoring regression of disease during neoadjuvant chemotherapy. Other uses include postlumpectomy margin assessment17,18 and evaluating patients with adenocarcinoma of an axillary node and an unknown primary.19
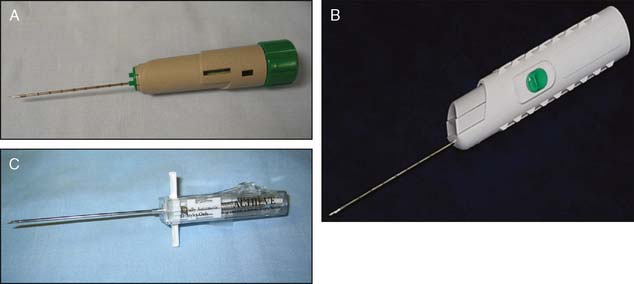
In concert with the improvements in imaging, there have been marked improvements in tissue acquisition. Percutaneous histologic tissue acquisition techniques currently in use, also referred to as minimally invasive breast biopsy, include large-core biopsy (typically 12–14 gauge) (Fig. 10-12A through C), including the Monopty and Maxcore by Bard and the Achieve needle by Cardinal Health; the vacuum-assisted biopsy (VAB) (typically 7–11 gauge), such as the Mammotome from Ethicon Endo-Surgery, the EnCor from SenoRx, and ATEC from Hologic (Fig. 10-13A through C), and larger tissue acquisition systems, such as the Halo from Rubicor and the en-bloc from Neothermia Corporation (Fig. 10-14A and B). Using smaller needles may risk the occurrence of higher false-negative rates. Fine-needle aspiration (FNA) techniques may be used for biopsy of axillary or internal mammary lymph nodes but are otherwise felt to be less desirable than histologic tissue acquisition techniques. The larger tissue acquisition systems (Rubicor and en-bloc) appear to offer little advantage over VAB for diagnostic procedures. Investigation of their therapeutic potential is ongoing.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree