Fig. 15.1
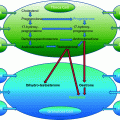
Forest plot of comparison: 1 Clomiphene citrate with gonadotropins (with or without mid-cycle antagonist) versus gonadotropins with GnRH agonists protocols in IVF and ICSI cycles, outcome: 1.1 live birth
There was a significant reduction in the incidence of OHSS (5 RCTs, 1559 women; OR 0.23, 95% CI 0.10–0.52) Compared to a typical clinic with 3.5% prevalence of OHSS using a GnRH agonist regimen, clomiphene citrate protocols would be expected to reduce the incidence between 0.8 and 1.8% (Fig. 15.1) [5]. The evidence from this review suggests that the use of clomiphene along with gonadotropins leads to similar pregnancy rates as those occurring after the use of gonadotropins alone, and there was a significant reduction in the incidence of OHSS (Fig. 15.2).
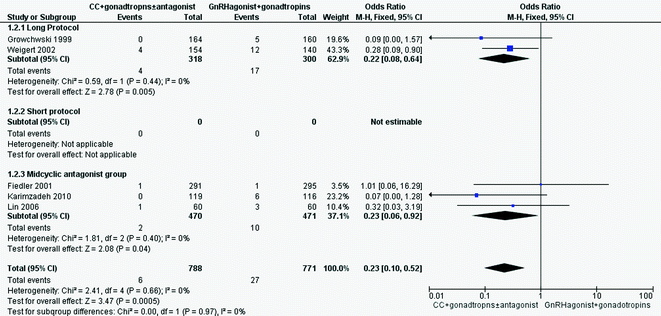

Fig. 15.2
Forest plot of comparison: clomiphene citrate with gonadotropins (with or without mid-cycle antagonist) versus gonadotropins with GnRH agonist protocols in IVF and ICSI cycles; outcome: ovarian hyperstimulation syndrome
Indication for Mild Stimulation Protocols: Combination of Clomiphene Citrate and Recombinant FSH or HMG
Patients Who Are at High Risk of Hyper-response
Personalization of treatment in IVF should be based on the prediction of ovarian response for every individual AFC (antral follicle count) and AMH (anti-Mullerian hormone) the most sensitive markers of ovarian [8] (Fig. 15.3), reserve identified to date, are ideal in planning personalized COS protocols. AFC was found to be significantly associated with AMH levels and the number of retrieved oocytes, with the number of follicles between 5 and 6 mm having the highest correlation to both endpoints. AMH (as a paracrine product of immature follicles) is a more direct measure of ovarian status compared with other endocrine reproductive hormones. AMH is primarily produced by the pre-antral and small antral follicles and correlates with the number of primordial follicles at the gonadotropin-independent stage of follicular development (Fig. 15.4) [8].
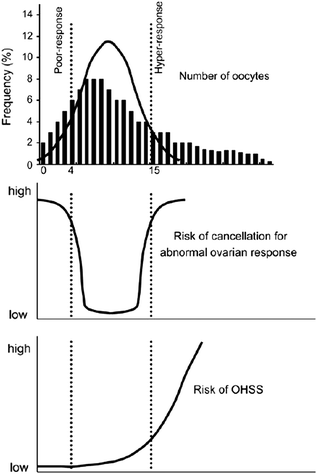
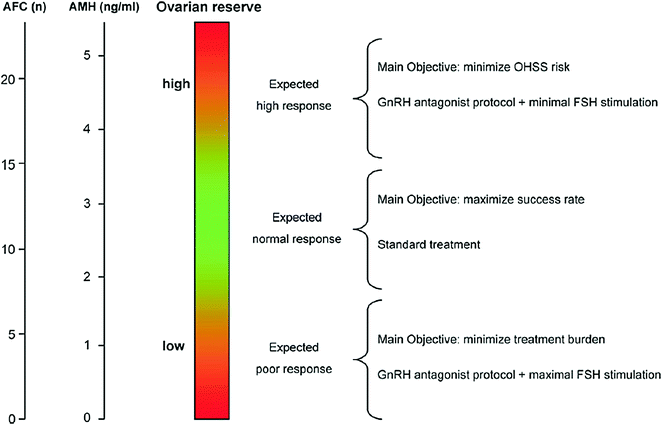

Fig. 15.3
Objective of the individualization of the treatment strategy would be possible to increase the percentage of patients with a number of retrieved oocytes considered appropriate, hence reducing the number of women at high risk of cycle cancellation and ovarian hyperstimulation syndrome (OHSS). Top of the figure: Bars indicate the actual frequency of retrieved oocytes as derived by La Marcal and Sunkara [8]. The line indicates the ideal frequency of retrieved oocytes, characterized by a very high percentage of women with an appropriate oocyte yield

Fig. 15.4
Ovarian reserve testing before the first IVF cycle would be permit to categorize patients as expected poor, normal, or hyper-responders. Since there is no evidence of superiority of one approach over another in the treatment of poor responders, the protocol associated with reduced discomfort and treatment durden should be preferred. In hyper-responder patients, one of the most important objectives of medical counseling is to prevent OHSS. Hence, the first-line protocol would be based on administration of low doses of FSH in a GnRH antagonist-based scheme. AFC, antral follicle count; AMH, anti-Mullerian hormone
High risk of hyper-response means high risk of ovarian hyperstimulation syndrome (OHSS) or had preliminary OHSS history. It is of great importance to accurately predict women who are likely to have a high response to COS as it is the main risk factor for OHSS [9]. Initial follicular recruitment and selection are undertaken by endogenous endocrine factors prior to starting the exogenous gonadotropin administration. Compared with the standard long GnRH agonist protocol, mild stimulation leads to a smaller number of growing follicles and this is undoubtedly an advantage in women with a high ovarian reserve and hence at risk of OHSS. In the study, recently [10], a modified therapeutic protocol with low gonadotropin doses and GnRH antagonist or clomiphene seems to be ideal for women at high risk of OHSS. [8] (Fig. 15.4). Mild/minimal stimulation offers an attractive option for patients who have experienced this complication in a previous treatment cycle, and it can reduce the incidence of OHSS in high-responder patients [11].
Polycystic Ovary Syndrome (PCOS)
Severe OHSS may occur particularly when the classical “long” protocol is applied to young, highly responsive women, and its incidence ranges between 2 and 6% of the IVF cycles in this kind of women [12]. PCOS patients usually have high number of AFC and high AMH level, and most of them are high risk of OHSS, especially severe OHSS.
Anticipated Poor Response
For anticipated poor response patients, treatment with mild stimulation protocol as likely to be beneficial reduced the treatment burden than the conventional GnRH agonist long protocol. According to published data, a cut-off value of AMH ranging between 0.7 and 1.3 ng/ml may be considered acceptable for the prediction of poor response in IVF. The recent European Society of Human Reproduction and Embryology Consensus Conference established a standardized definition of poor ovarian response as the retrieval of 4 oocytes following a standard IVF protocol, i.e., following maximal stimulation [13]. The most frequently reported cut-off values of AFC for prediction of poor response ranged between 5 and 7 [14, 15]. The advantages of mild stimulation protocols particularly apply to low-response patients regarding cost, convenience, and success rates. High-dose gonadotropin is unused for the poor response, and mild/minimal stimulation may be an optional protocol. Anticipated poor response would never be compensated by an increase in the exogenous FSH over the maximal dose. Accordingly, different studies performed on women anticipated to be poor response on the basis of low AMH or AFC showed that increasing the FSH dose was ineffective for preventing a negative ovarian response in those women [16–18].
Target Number of Oocyte Is 5–8
Conventional control ovarian stimulation protocols aim to get many oocytes to compensate for inefficiencies in laboratory procedures and to generate several embryos for transfer. With the development of the laboratory techniques, less oocytes were needed for IVF treatment and less embryos were transferred. Five to eight oocytes will be enough for most people.
Patients Who Are Afraid of Long Time of Injection
The injection duration of mild stimulation time usually is 7–10 days, much less than conventional protocol of 20–25 days. Some studies suggest that women who receive milder approaches in ovarian stimulation could be more prone to face a new treatment attempt compared with women receiving a standard protocol: In fact, the psychological burden of treatment is one of the most frequent causes of dropout, and a significantly lower dropout rate was observed in more patient-friendly “mild” stimulation programs [19, 20].
Low Score of Oocytes or Embryos by Morphology for the Last Conventional Ovary Stimulation Cycle
Several reports [21] demonstrated that the chromosome error rate was higher when more oocytes were retrieved. This study demonstrated that a high oocyte yield resulted in more chromosomally abnormal embryos, particularly in younger women. Study by Katz-Jaffe [22] found similar results, demonstrating that the likelihood of segregation errors seen in early embryo cleavage states is reduced with mild stimulation. A randomized trial concerning the chromosomal constitution of human embryos following mild ovarian stimulation for IVF showed a significantly higher proportion of euploid embryos compared with conventional stimulation, suggesting that through maximal stimulation, the surplus of obtained oocytes and embryos is of lower quality [23]. That means, despite “mild” stimulation obtained significantly fewer oocytes and embryos, both regimens (conventional long protocol and mild stimulation) finally generated the same number of chromosomically normal embryos. This observation suggests that the reduced pharmacological interference with ovarian physiology could generate oocytes of better genetical quality.
Patients Who Could Accept for FET Instead of Fresh Embryo Transfer
One of the main side effects of CC is the effect of the thickness of the endometrium. Endometrial thinner than conventional program may affect embryo implantation rate. Additionally, because gonadotropins may counterbalance the undesired anti-estrogenic effects of the clomiphene on the endometrium [7, 10] which has been held responsible for the relatively low embryo implantation rates observed, this combination might lead to improved pregnancy rates compared with clomiphene alone. In the clomiphene combined gonadotropin cycle, patients with proper thickness of the endometrium (≥8 mm) also can have fresh embryo transfer.
Prefer for a Cheaper Treatment Cycle
A mild ovarian stimulation using clomiphene combined with gonadotropin is undoubtedly associated with a lower medication consumption and with a lower cost for purchasing drugs than either the conventional agonist long protocol or the antagonist protocol.
No Pre-ovulation in the Past
HCG day slightly higher LH levels may increase pre-ovulation. Patients who had a pre-ovulation history are unfit for the program of clomiphene combined with gonadotropin.
IVF Cycle Management
- 1.
Preparation before ovary stimulation protocol is essential. The baseline evaluation should be taken place in the first three days of menstruation. If baseline levels of estradiol (less than 183.5 pmol/L or 50 pg/ml), and serum FSH less than 20 IU/L had been achieved, trans-vaginal ultrasound on the third day of menstruation. If the antral follicles diameter is among 4–8 mm, the stimulation procedure could be started.
- 2.
Clomiphene citrate 50 mg/day (Medochemie Ltd.) was administrated orally with an extended regimen from cycle day 3 until the day of HCG trigger. At the same time, the right dosage of gonadotropin (HMG or recombinant FSH 75–150 IU per day) was added in injections. After starting 5 days of co-stimulation, on day 8 of the menopause, serum LH/E2 and trans-vaginal ultrasound should be monitor for the ovarian response (Fig. 15.5).
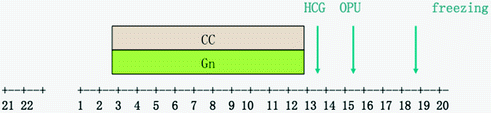
Fig. 15.5
IVF treatment workflow. CC, clomiphene citrate; Gn, gonadotropin
- 3.
The dosage of gonadotropin could be adjusted according to the results of the serum hormone and follicle size until the triggering day. GnRH antagonists were not routine used because LH suppression was already obtained by the extensive clomiphene citrate regimen except that the serum LH is higher than three times of the basal day 3 LH with the small follicle size that could not be trigger. If the follicle size is small and the LH increased, LH suppression may not be attained by using clomiphene citrate alone, and GnRH antagonists should be added and are still effective to prevent the LH surge and the pre-ovulation.
- 4.
When dominant follicle diameter reached 14 mm, indomethacin could be used to prevent the pre-ovulation(0.1 g per day by rectal administration).
- 5.
When at least two leading follicles size reached 18 mm, with appropriate serum E2 levels, ovulation triggering with human chorionic gonadotropin (HCG) or GnRH agonist was routinely performed (Ovidrel 250ug Hst, EMD Serono, Inc.). On the morning of the trigger day need to monitor LH\P\E2 data, clomiphene citrate was used until the trigger day.
- 6.
Trans-vaginal ultrasound-guided oocyte aspiration (OPU) was performed approximately 36–37 h after HCG injection.
- 7.
Either IVF or ICSI was performed according to the clinical indication.
- 8.
On the third day of OPU(D3), fresh embryo transfers performed or delayed vitrified–warmed embryo transfer according to the thickness of endometrial, embryo-transfer procedures were performed under abdominal ultrasound guidance using a soft catheter (Sydney, Cook, Australia). No more than two embryos were transferred in order to avoid triplet pregnancies.
- 9.
Frozen embryo transfer (FET) were usually performed during the next-month period following the oocyte retrieval.
- 10.
The luteal phase was supported by administering 20 mg/d Dydrogesterone Tablets (Abbott Healthcare Products B.V.) and Utrogestan 400 mg/d (Progesterone Capsules, Besins Manufacturing Belgium) for 14 days. Pregnancy was assessed by serum hCG assay after 14 days from embryo transfer and then confirmed when a gestational sac was visualized at vaginal US after two to three further weeks.
- 11.
About thawing cycle management. Thawing cycles were performed on a nature cycle or control ovarian stimulation or hormone replacement cycle: Trans-vaginal ultrasound was adjusted to the follicle size or the thickness of endometrium. HCG was used when a dominant follicle reaching 18 mm diameter. A maximal number of two thawed embryos were transferred three days later after the ovulation, or the fifth day of the progesterone used in the hormone replacement cycle;
Our Preliminary Data of Mild Stimulation of Clomiphene Combined with Gonadotropin
Preliminary Data for Poor Responders or Slow Responders
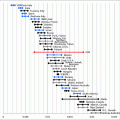
Mild stimulation of clomiphene combined with gonadotropin was used for 63 patients that were poor responders or slow responders in the pretreatment IVF cycle in 2010–2011 in our center. The average age is 32.5 ± 4.2 years, basal FSH average is 8.6 ± 3.2 IU/L, average antral follicle count is 5.9 ± 3.2, average stimulate duration is 8.3 ± 1.8 days, average total Gn dose is 831.0 ± 287.8 IU, average levels of estrogen are 8948.2 ± 5320.2 pmol/L on the HCG trigger day, progesterone levels are 3.6 ± 2.1 nmol/L, and mean endometrial thickness is 7.0 ± 1.5 mm (Table 15.1). The average number of oocytes retrieved per cycle was 5.2 ± 3.2, the average number per embryos transferred was 1.6 ± 0.5, the cumulative clinical pregnancy rate was 39.6%, cumulative live birth rate was 35.8%, and pregnancy loss rate was 4.8% (Table 15.2).
Table 15.1




Baseline characteristics of the patients (n = 63)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree