Fig. 9.1
Recommended intakes for pregnant women expressed as percentages of the reference intake values for nonpregnant women. The recommended intakes of iron and folate show great increases of 100 and 50 %, respectively, compared with those for the nonpregnant state, while energy needs, calcium, and vitamin D show a smaller change (Adapted from Koletzko et al. [26])
Micronutrient deficiencies have been associated with significantly high reproductive risks, ranging from infertility to fetal structural defects and long-term diseases. On the other side, healthy dietary patterns and micronutrient supplementation, particularly during the periconceptional period, are related to improved birth outcomes, probably through alterations in maternal and fetal metabolism due to micronutrient role/involvement in enzymes, signal transduction, transcription pathways, oxidative stress, and epigenetic modifications [11]. Since different pregnancy stages represent a continuum, from the preconception to the postpartum period, an injury acting before conception or in early pregnancy may have long-lasting effects on the well-being of the mother and the fetus and may further influence the health of the baby at a later age.
It has been estimated that more than two billion people in the world today suffer from micronutrient deficiencies caused largely by a dietary deficiency of vitamins and minerals. Nutritional deficiencies are known to lead to adverse pregnancy outcomes involving the great obstetrical syndromes such as preterm delivery, small for gestational age (SGA) offspring, and maternal hypertensive disorders as well as to long-term effects in the offspring. The chapter will focus primarily on the following outcomes: intrauterine growth restriction, preeclampsia, and preterm delivery.
9.2 Micronutrient Malnutrition
Contrary to previous thinking, micronutrient malnutrition is not uniquely the concern of poor countries. While micronutrient deficiencies are certainly more frequent and severe among disadvantaged populations, they do represent a public health problem also in most industrialized countries where obesogenic unbalanced diets have a major impact on macro- and micronutrients. Worldwide micronutrient intakes do not fit pregnancy requirements.
Since 1989 it has been shown that dietary surveys of pregnant women in industrialized countries consistently demonstrate Fe intake well below current recommendations. A recent review [6] summarizes the best available evidence related to the food-derived vitamin and mineral intakes of pregnant women in developed countries demonstrating that folate, iron, and vitamin D intakes are consistently below nutrient recommendations in each geographical region. In particular, despite folate recommendations varying across geographical regions, average folate intakes in all regions were between 13 and 63 % lower than recommendations. Similarly, iron intakes reported by pregnant women were below nutrient recommendations in almost all developed regions. However, these results underestimate the number of pregnant women who met national pregnancy micronutrient targets on the basis of combined food and supplement intakes.
Moreover, many micronutrients with important roles during pregnancy (e.g., iodine) were not commonly reported within the included studies, and thus, nutritional adequacy could not be evaluated. In the last 10 years, median urinary iodine below the recommended values has been reported in pregnant women of many countries in the world (Argentina, Greece, Italy, Wales, Ireland). However, there are no studies about a potential role of iodine in the great obstetrical syndromes.
In fact, although a balanced diet is generally accessible in industrialized countries, a switch to highly processed energy-dense but micronutrient-poor foods is likely to adversely affect micronutrient intake and status.
9.3 From Requirements to Recommendations: The Strange Case of Pregnancy
The ideal definition of a physiological requirement is the amount and chemical form of a nutrient that is needed systematically to maintain normal health and development without disturbing metabolism of any other nutrient. The physiological requirement is very difficult to be estimated, and for this reason micronutrient requirement is defined as the minimum amount needed by an individual to avoid deficiency.. However, micronutrient recommendations are designed for populations or subgroups of populations, and they are defined as intakes of micronutrients sufficient to meet the requirements of the majority of healthy individuals (generally 97.5 %) of that population or group.
Many countries have developed recommendations for intake of micronutrients in the normal diet. However, there is considerable variation in the recommended micronutrient intakes used by countries in particular within Europe, partly due to different methodologies and concepts used to determine requirements and different approaches used to express the recommendations. The EURRECA Project was designed in Europe. Specifically, the EURopean micronutrient RECommendations Aligned (EURRECA) Network of Excellence has worked to provide an evidence-based framework for establishing micronutrient requirements. EURRECA is a network of excellence funded by the European Commission and established to address the problem of differences between countries in micronutrient recommendations [15].
First of all the EURRECA network developed a selection process to identify a list of micronutrients to focus on. The prioritization of micronutrients was based on the availability of new scientific evidence, the public health relevance, and the heterogeneity of the recommendations [10]. Ten micronutrients were identified as most urgently in need of review: vitamin D, iron, folate, vitamin B12, zinc, calcium, vitamin C, selenium, iodine, and copper. Of these, vitamin C, vitamin D, folate, calcium, selenium, and iodine were the nutrients showing a higher prevalence of inadequate intakes in Europe. In particular, a lack of harmonized and evidence-based recommendations was found for the supply of vitamin D, folate, and iron during pregnancy, lactation, and infancy [21].
9.3.1 Identification and Reference Values of Micronutrients
Once the most important micronutrients are identified, valid information on micronutrient intakes and status of a population (Fig. 9.2) is fundamental for deriving micronutrient reference values.
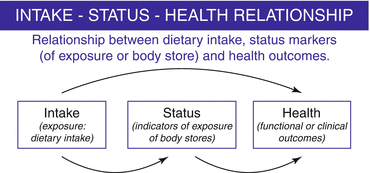

Fig. 9.2
Intake–status–health relationship
For this purpose, biomarkers of micronutrient status need to be identified for each micronutrient. Examples of types of status biomarkers include plasma concentrations, the size of body pools, enzyme levels and activities, urinary excretion, and a range of other biochemical and/or functional indicators which have varying degrees of specificity and sensitivity (Table 9.1). Indeed, nutritional status, when applied to specific micronutrients, reflects a continuum, from short-term (hours) exposure (to long-term (months/years) effects. Moreover, a rigorous selection process needs to be carried out also for identifying health outcomes of interest for each micronutrient: for example, EURRECA identified the most relevant health outcomes by determining the number of hits that emerged in preliminary searches of the literature combined with the opinion of scientific nutritional experts [17].
Table 9.1
The reliable biomarkers or indicators of status of the main micronutrients
Micronutrient | Biomarkers |
|---|---|
Folate | Erythrocyte folate |
Serum/plasma folate | |
Serum/plasma total homocysteine | |
Vitamin B12 | Serum/plasma vitamin B12 |
Serum/plasma methylmalonic acid (MMA) | |
Iron | Hemoglobin |
Serum ferritin | |
Serum transferrin receptor | |
Vitamin D | Plasma 25-hydroxyvitamin-D [25(OH)D] |
Iodine | Urinary iodine excretion in 24 h |
Serum thyroid-stimulating hormone (TSH) |
Finally, there are different approaches for deriving reference dietary values. The association approach addresses the dose–response relationship between dietary micronutrient intake and body status or health outcomes. The factorial approach addresses micronutrient losses and maintenance, absorption/bioavailability, and additional requirements for specific life stages. Currently, most of the available studies evaluate the role of different nutrients such as micronutrients in pathologies and health outcomes. However, foods are not consumed in isolation. An overall dietary pattern might have a greater effect on health than a single food or nutrient. Methods most often used to characterize diet and dietary pattern have been based either on a priori knowledge (hypothesis-oriented DP) or derived from food frequency questionnaires using data-driven dimension reduction techniques, such as principal components analysis (PCA) (empirically derived DP).
9.3.2 Genetics and Individualization
It is important to recognize the multiple ways by which a nutritional deficiency may arise. First, a deficiency can occur as a consequence of an inadequate dietary intake of an essential nutrient. But a secondary deficiency may occur even if the dietary content of the essential nutrient appears to be adequate. Conditioned deficiencies can arise through several mechanisms as genetic factors, nutritional interactions, effects of drugs or chemicals, or diseases (as diabetes or hypertension).
Critical to this consideration will be the need for a better understanding of the influence of maternal and conceptus genotype on their susceptibility to the toxicity as well as the deficiency of essential micronutrients.
In addition, a new reference intake, the tolerable upper intake level (UL), has also been established to assist an individual in knowing how much is “too much” of a nutrient. Where adequate scientific data are available, determining tolerable upper intake levels for each micronutrient in specific population subgroups should be a primary goal.
9.3.3 Vulnerable Groups: Pregnant Women
A critical step is also to identify vulnerable population groups who are at greater nutritional risk: i.e., population groups in a healthy population having a higher requirement. Pregnant women are considered a “vulnerable group,” as their nutritional requirements increase significantly to support fetal and infant growth and development as well as maternal metabolism and tissue accretion.
From a nutritional point of view, pregnancy can be simplified by a three-compartment model, i.e., mother/placenta/fetus. Each of them has a different metabolism and they interact. Apart from maternal diet and from metabolism of the various organs, placenta transport function determines the composition of the umbilical cord blood providing nutrients and oxygen to the fetus. Fetal growth is regulated by the balance between fetal nutrient demand and maternal–placental nutrient supply. Maternal nutrition and metabolism, uteroplacental blood flow, size, and transfer capabilities of the placenta all determine the maternal–placental supply of nutrients. On the other hand, pregnancy is a dynamic state, during which adjustments in nutrient metabolism evolve continuously as the mother switches from an anabolic condition during early pregnancy to a catabolic state during late pregnancy. Consequently, qualitative differences in dietary requirements exist during early and late pregnancy [3] (Fig. 9.3).
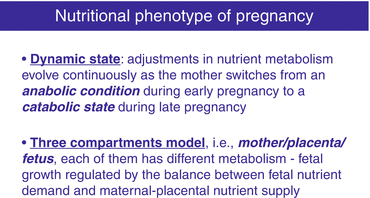

Fig. 9.3
Nutritional phenotype of pregnancy
Dietary assessment using food frequency questionnaire (FFQ) during pregnancy may be complex. Some recommendations should be applied when dietary patterns during pregnancy are analyzed: (1) to employ a validated food frequency questionnaire designed for use in pregnancy, (2) to consider the special role exerted by mineral and vitamin supplements in this particular population group, (3) to adequately select the time in which dietary data are collected, and (4) to adjust the results for lifestyle and educational characteristics [34].
9.4 Preeclampsia
Preeclampsia is one of the leading causes of maternal morbidity and mortality and preterm delivery in the world. Nutritional interventional studies to prevent hypertension and different end-organ diseases grouped under the same syndrome are biased by the different prevalence of clinical phenotypes that are the results of various pathological process. Abnormal placentation with release of anti-angiogenetic factors in the maternal circulation, together with a predisposed maternal phenotype, seems to be involved. In particular, preeclampsia is associated with an increased inflammatory state, but the role of maternal nutrition in influencing the risk of developing preeclampsia is unclear.
9.5 Preterm Delivery
Low body mass index (BMI) before pregnancy and inadequate body weight gain of pregnant women in the second and third trimesters have been shown to be significantly associated with spontaneous preterm delivery in the USA and in Europe. Moreover, recent data obtained in 66,000 women in Norway showed that high scores on the “prudent” dietary pattern in the first months of pregnancy are associated with significantly reduced risk of preterm delivery hazard ratio [16]. This dietary pattern is characterized by intake of vegetables, fruit, whole grains, fish, and water, suggesting higher intake of micronutrients.
In this context, several studies suggest that multivitamin and mineral use before and during pregnancy may potentially prevent preterm birth. However, there is still not enough evidence to support multivitamin supplementation for reduction of preterm delivery.
A trial by Catov et al. [8] demonstrates a reduced risk of preterm delivery and small for gestational age in a cohort of 1,823 women who reported periconceptional multivitamin use, even if this finding was limited to women with a BMI <30. In 2011 data from more than 35,000 women in the Danish National Birth Cohort [9] confirmed that women who reported periconceptional multivitamin use had a reduced risk of preterm birth and a reduced risk of SGA (<5th percentile). The risk of an SGA birth was reduced in multivitamin users regardless of their prepregnancy BMI, with the strongest association in regular users in the post-conception period. However, these findings should be interpreted with caution because multivitamin use also correlated strongly with other lifestyle factors.
Although mechanisms that may link periconceptional multivitamin use to preterm delivery are not understood, impaired placentation seems to be implicated. Placentation is characterized by vascular remodeling, oxidative stress, inflammation, and rapid cell division, all of which may be affected by nutritional status. During pregnancy, oxidative stress has been implicated not only in the pathophysiology of preeclampsia but also of preterm birth leading to poor birth outcome. Moreover, hyperhomocysteinemia, as a consequence of altered micronutrients like folic acid and vitamin B12, is associated with increased production of reactive oxygen species that generate oxidative stress. One case–control study related the preconception sufficiency of vitamins B6 and B12 in maternal serum to a 50–60 % reduced risk of a PTB. Nevertheless a large prospective national birth cohort study in Norway did not find a protective effect of dietary folate intake or folic acid supplementation on spontaneous preterm delivery in uncomplicated pregnancies [33].
9.6 Low Birth Weight and Intrauterine Growth Restriction
Mothers in low-income countries frequently have inadequate micronutrient intakes. They often consume inadequate levels of micronutrients due to limited intake of animal products, fruits, vegetables, and fortified foods. Low birth weight (LBW) is common in undernourished populations in low- and middle-income countries, predominantly because of intrauterine growth restriction. Micronutrient supplementation and fortification are currently being used as strategies to improve nutrition even in resource-poor settings since many girls and women are chronically undernourished. Many trials have assessed the effect on birth weight of giving women of low socioeconomic status micronutrient supplements during pregnancy.
In 2001 the Pune (India) Maternal Nutrition Study showed the lack of association between size at birth and maternal energy and protein intake but strong associations with folate status and with intakes of foods rich in micronutrients that suggested that micronutrients may be important limiting factors for fetal growth in this undernourished community [30]. However, in another trial performed in the Indian population, a daily snack providing additional green leafy vegetables, fruit, and milk before conception and throughout pregnancy had no overall effect on birth weight. Subgroup analyses indicated a possible increase in birth weight if the mother was supplemented >3 months before conception and was not underweight.
In the USA a prospective cohort study of 1,116 low-income and minority pregnant women showed that suboptimal maternal calcium intake and vitamin D status might affect fetal growth [32]. Studies in India have shown a low dietary consumption of vitamin B12 due to dietary pattern of vegetarianism and poor consumption of milk and milk products. In vegetarian Indians, deficiency of vitamin B12 is commonly seen, while concentrations of folate are adequate. Together with folic acid, vitamin B12 is also an important determinant of fetal growth and development. During pregnancy, the increased requirement of folic acid is met with supplementation, while vitamin B12 remains untreated and possibly deficient. In addition to vitamin B12 and folate deficiencies alone, there may be adverse birth outcomes associated with unbalanced vitamin B12 and folate intakes or status during pregnancy. Folate to vitamin B12 ratio was correlated to neonatal anthropometric measures. Imbalance in the maternal micronutrients with increasing ratios of folate to vitamin B12 was associated with an increase in plasma homocysteine and lowering of neonatal birth weight, birth length, head circumference, and chest circumference, while no significant association to other anthropometrics was observed.
In 2009 a meta-analysis on original data from 12 randomized, controlled trials concluded that compared with iron–folic acid supplementation alone, maternal supplementation with multiple micronutrients during pregnancy in low-income countries resulted in a small increase in birth weight and a reduction in the prevalence of LBW of about 10 %. The effect was greater among women with higher BMI [18].
A recent meta-analysis of 21 randomized controlled trials on the effects of multiple supplementation on pregnancy outcome found that low-/middle-income women who received multiple supplements had a significant reduction in the risk of SGA offspring compared with women who received iron/folate supplements alone also in developing countries [19]. However, this study failed to show a significant impact on any of the other outcomes of pregnancy. The Generation R Study in Rotterdam provided evidence that low adherence to the Mediterranean diet in the periconceptional period significantly increased the risk of intrauterine growth restriction [37].
9.6.1 Iron Requirements in Pregnancy and Small Fetuses
Indeed, current studies indicate that micronutrient deficiencies might increase the risk of preterm delivery and impair fetal growth also in industrialized countries. Specifically, iron depletion in pregnancy has been associated with maternal anemia, reduced fetal and neonatal iron stores, increased risk of SGA offspring, and preterm delivery. Placental iron transport against a concentration gradient firstly allows to minimize the effect of fetal deficiencies by upregulating the proteins involved in iron transfer.
Iron deficiency is one of the most prevalent nutritional deficiencies worldwide and often leads to iron deficiency anemia (IDA). An inadequate dietary intake is the leading cause of IDA development, although other physiological and pathologic conditions, including impaired absorption or transport of iron, physiological losses, or chronic blood loss secondary to disease, could contribute as well. Worldwide, the prevalence of anemia (defined as a hemoglobin level <11 g/dl) has recently been estimated around 38 % in pregnancy, translating into 32 million pregnant women (Nutrition Impact Model Study 1995–2011) [36], with a small reduction compared with the 1990s. Even in high-income countries, it is estimated that approximately 45 % of women have serum ferritin <30 μg/l, indicating small or absent iron reserves, while only 15–20 % have ferritin >70 μg/l, which nearly balances the net iron loss during pregnancy.
From the meta-analysis of Blumfield et al. [6], iron intakes reported by pregnant women in developed countries were below the nutrient recommendations in all regions except the UK.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree