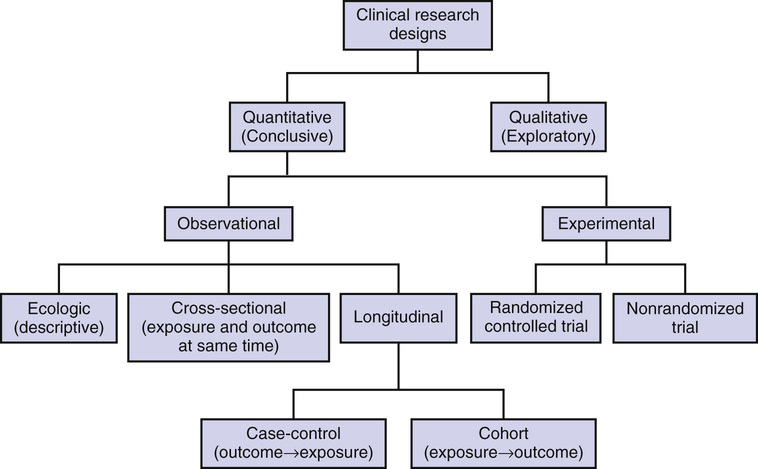
Antony Bayer The difficulty of undertaking research involving older people tends to be exaggerated. It is wrongly assumed that too many will have significant comorbidity leading to a poor signal-to-noise ratio, an unacceptably high risk of adverse events, inability to complete necessary assessments, poor compliance, and high dropout rate. This can translate into arbitrary, unscientific, and unnecessary upper age limits. However, many of the changes commonly attributed to aging are typically due to reasons other than chronologic age, notably physical and cognitive comorbidities leading to frailty and psychosocial factors, such as relative lack of education and cigarette smoking. Furthermore, it is often older adults who have the greatest morbidity and mortality associated with the condition under study and who therefore will have the greatest absolute benefit from any effective intervention. Ill-informed beliefs about the supposed high risk of developing mental incapacity and perceived low life expectancy after age 65 are sometimes used to justify the exclusion of older people from longitudinal studies because it is wrongly assumed that few will stay the course. In reality, the annual incidence of dementia in those older than 65 years is about 1%, and life expectancy at age 65 in England currently averages between 18 and 21 years. Ethical concerns about experimenting on older populations, who are considered vulnerable only on the basis of chronologic age, demonstrate misguided paternalism of younger research workers and ignores the older person’s right to autonomous decision making. Most of even the oldest old will have no significant cognitive impairment and will generally have the capacity to make an informed decision about participation. The consequences of excluding older people from therapeutic research, where they are left to receive treatment in the absence of evidence-based trials or are denied drugs because they have been untried in their age group, might be considered especially unethical1 and imply that clinicians have a duty to encourage actively their inclusion in clinical trials.2 Guidance to promote research with older people has been developed by the European Forum for Good Clinical Practice,3 and greater involvement of older people in clinical trials is also endorsed by regulatory authorities in Europe and the United States who evaluate drugs for registration.4,5 All researchers should be careful, therefore, that ageist attitudes do not influence their research design and practice, and funding bodies and research ethics committees should challenge unnecessarily restrictive entry criteria, including inappropriate upper age limits.6 The optimum choice of design to study aging and age-related conditions and to understand the mechanisms underlying change and their consequences will depend on the research question to be answered (Figure 6-1). Qualitative studies, ecologic studies using available data, and quantitative studies using cross-sectional, case-control, and cohort designs will help generate hypotheses. These can then be tested in experimental studies using randomized controlled trial designs. Each design presents its own challenges and limitations. Qualitative research has its roots in anthropology and sociology and is an umbrella term for a heterogeneous group of methodologies with different theoretical underpinnings.7 They aim to gain an in-depth understanding of peoples’ behavior by exploring their knowledge, values, attitudes, beliefs, and fears. This allows subjects to give richer answers to real-world issues and allows the researcher to explore the full complexity of human behaviors, thus providing detailed insights that might be missed by other methods. For example, it may illuminate the reasons behind patients’, carers’, and clinicians’ decisions about management, and therefore inform future policy developments,8,9 or help characterize important issues such as abuse or risk management that may be difficult to quantify.10,11 Qualitative studies are hypothesis-generating rather than hypothesis-testing studies, but results can identify specific issues that need to be tested using quantitative methods or can help explain the outcomes of experimental studies. Thus, the two methods can usefully complement each other, and increasing numbers of studies are using mixed methodologies (e.g., a study trying to understand the attitudes of older adults toward enrollment into cancer clinical trials).12 Samples in qualitative research tend to be small and labor-intensive, with data collected usually by direct observation or active participation in the setting of interest, or by in-depth individual interviews (unstructured or semistructured), focus groups (guided group discussions), or examination of documents or other artifacts. Other methods used in qualitative research studies include diary methods, role playing and simulation, narrative analysis, and in-depth case studies. Although potential areas of interest may be identified beforehand, there is no predetermined set of questions, and subjects are encouraged to express their views and ideas at length. Rather than formal sample size calculations, numbers of participants may be decided by analyzing interviews alongside data collection, which is stopped when no new themes are emerging (saturation). Sampling tends to be purposive rather than comprehensive or random, deliberately aiming to reflect a specific range of experience and attitudes judged to be of likely relevance to the research question. The results are analyzed by exploring the content and identifying patterns or themes, often through an iterative process allowing meaning to emerge from the data, rather than by the deductive statistical approach of quantitative methods. Critics of qualitative analysis are concerned that it is too influenced by the views and attitudes of the researchers when they are collecting and analyzing data, thus introducing unacceptable bias and problems with generalizability and reproducibility of findings. Qualitative research can be challenging with older people but, because it can be less intrusive than more structured quantitative methodologies, it may be especially suited to those who are frail. They may be unable or unwilling to take part in lengthy interviews because of communication deficits or fatigue, and several shorter interviews may be more practical. Focus groups may work best with just four or five older participants and need a skilled facilitator to ensure a high level of participant interaction. Extra effort is needed to ensure representative samples and support those who are less confident, easily fatigued, or have cognitive or physical deficits. Participant or nonparticipant observation may be especially useful in institutional settings, but time must be given to establish trust with the researcher if residents and staff are not to feel threatened. Assurances of confidentiality and commitment from management are essential. However, once trust has been established, attrition rates tend to be low, because participation tends not to be burdensome.13 Ecologic studies use available data to characterize samples and generate hypotheses, although evidence for causality is generally weak. Data may be aggregated, such as census data and records of disease incidence by hospital, or individual, such as hospital discharge summaries or death certificates. Because the data are already available, there are advantages of speed and economy and the impact of factors operating at a population level (e.g., improved access to education, banning smoking in public places) may be difficult to measure at an individual level. However, measures may not be comparable over time or place, quality is always outside the researcher’s control, and the available data may be selective. Many official statistics that are broken down by age will lump all those older than 65 years together or will only report information on adults of working age. When older people are included, they often exclude those not living in the community and those with cognitive impairment. Nevertheless, temporal data, such as the effect of daily variations in air pollution or temperature on mortality of older adults, where individual confounding factors remain constant over time, can provide robust evidence suggesting a causal effect. Ecologic data is also of value in studying the effects of early life factors on later health or disease on “life course epidemiology.”14 Cross-sectional studies record information over a short period of time and are suited to report prevalence and the relationship between variables and age or dependency. They are relatively fast and simple to conduct because each subject is examined only once, and several outcomes or diseases can be studied simultaneously. For example, data from the Health and Retirement Study of 11,000 adults aged 65 years or older (representing the 34.5 million older Americans) highlighted the important finding that common geriatric conditions (e.g., cognitive impairment, falls, incontinence) were similar in prevalence to common chronic diseases in older adults, such as heart disease and diabetes, and were strongly and independently associated with dependency in activities of daily living.15 However, cross-sectional studies give no information about incidence or causality and are of limited value when studying rare conditions or acute illness. Data can be presented as the mean value for each age group, or age can be used as a continuous independent variable in a regression analysis, with the outcome of interest as the dependent variable. Associations can be confounded when the variable of interest affects the survival of subjects, with selective mortality leading to a survival bias. Misinterpretation can also arise from birth cohort effects, with associations and differences not arising due to age differences but due to the era in which people were born and brought up and to changes in exposure to environmental risk factors. Sometimes such differences from one generation to the next are of particular interest, and a time series design may then be appropriate, with sequential samples of a particular age group being studied every few years. The Cognitive Function and Ageing Studies (CFAS) 1 and 11, for example, were conducted 2 decades apart using the same diagnostic methods in the same older age group living in the same geographic areas and demonstrated a cohort effect in dementia prevalence, with later born populations having a lower risk than those born earlier in the past century.16 Selection of subjects needs to ensure that they are well matched at each time point, and methodologies need to be identical, to ensure that differences are solely due to temporal changes and not to selection bias. Case-control studies choose groups with (cases) and without (controls) the outcome of interest and look back at what different exposures they might have had to identify possible risk factors. They have been widely used in genetic epidemiologic studies (genome-wide association studies or GWAS) to identify susceptibility (risk) genes—for example, in Alzheimer disease.17 They are the best design to study uncommon conditions. Because they are efficient in use of time and money and collect much relevant information on targeted individuals. Case-control studies may be nested within cohort studies, with a subset of matched controls being selected from within the cohort and compared to the incident cases of the condition of interest. Bias can be introduced when cases and controls differ in ways other than just the outcome of interest (selection bias) or when cases are not typical (representativeness bias). Given the increasing heterogeneity characteristic of aging, bias can be a significant problem, and care needs to be taken to match cases and controls well. Recall bias may arise because cases are able to remember events better because of their significance or, unintentionally, they may be prompted to remember by investigators, who should therefore be blinded as to whether the person is a case or control when assessing exposures. People who have died do not make it into case-control studies, and their representatives are likely to be less reliable than people themselves at remembering exposures, introducing a potential survival bias. Although case-control studies can play a pivotal role in suggesting important associations, as in the original studies linking cigarette smoking and lung cancer,18 confounding can also lead to highly misleading conclusions, as in the observational studies of combined hormone replacement therapy and cardiovascular disease in postmenopausal women.19 In a cohort or longitudinal study, a group of subjects are followed over time as they age to determine who develops a particular outcome or the rate at which a variable changes. In addition to risk, the number of people who actually develop the outcome of interest can be calculated (incidence). Inevitably, such studies take a long time and often require a large sample size—the rarer the outcome, the larger the sample needs to be—and are therefore expensive. The frequency of testing needs to be decided based on the rate of change, precision of the measures being used, available resources, and stamina of researchers and research subjects. Analysis of longitudinal data by slope analysis or other techniques requires specialist knowledge. Prominent cohort studies relevant to old people include the Baltimore Longitudinal Study of Aging,20 Rotterdam Study,21 and Caerphilly Cohort.22 The UK Biobank has recently recruited a half-million people aged 40 to 69 years, all of whom have completed a very wide range of baseline assessments and who will now be followed long term (with some having state of the art imaging) to investigate risk factors for the major diseases of middle and old age. This resource is available for use by all bona fide researchers for all types of health-related research that is in the public interest.22a Recall bias is avoided in cohort studies because subjects are enrolled before the outcome(s) and sequence of events can be more clearly established, although the possibility of reverse causality must always be considered. Cohort effects are minimal because all the subjects are generally from a single birth cohort. Ideally, longitudinal aging studies would follow subjects from birth to the grave but this is unlikely because they would then outlive the research team. When age range in a longitudinal study is wide, cohort effects can be identified by plotting rates of change within age groups and seeing if the plots join up smoothly (a true age effect), or are a disjointed group of line segments similar to that often seen in repeated cross-sectional studies. Potential bias may arise when outcomes are not measured or not recorded in a consistent fashion over time, with small changes in methodology, such as new equipment, a change in assay technique, or differences in study personnel appearing to suggest age-related changes (detection bias). Ensuring a common period of training for all involved in the research, with periodic refresher courses and measures of inter- and intrarater reliability, can minimize problems, but researchers must stay alert to possible methodologic errors throughout data collection and analysis. Important outcomes may be missed if follow-up is too short or too long, so that subjects might die before they are reassessed. Inevitably, some subjects will drop out or be lost to follow-up (excursion bias), and there are various approaches to dealing with missing data by imputing values based on available records. A clinical trial is the methodology of choice to examine causality, with the randomized controlled trial (RCT) acknowledged as the gold standard for experimental design. The Standard Protocol Items: Recommendations for Intervention Trials (SPIRIT 2013) provides a checklist and explanation of recommended items to include in trial protocols.23 In an RCT, the researcher controls exposure to a single variable, the risk or treatment, by randomly assigning subjects to one group (intervention) or another (control, often involving a placebo intervention), and all subjects are then followed up to determine the outcome. When an effective intervention exists already, a placebo control is unethical, and the new experimental intervention is then compared against an active control (the current standard of care). In rare cases, when the size of the treatment effect relative to the expected prognosis is dramatic, randomization may not be necessary, or ethical and historical controls (apparently similar former patients) may be used.24 Parallel group RCT designs are generally preferred, with intervention and control groups being treated simultaneously. Thus half the subjects receive treatment A (intervention) and the other half receives treatment B (control). In a crossover design, subjects swap groups halfway through the study (half the subjects receiving treatment A are followed by treatment B, with the other half receiving treatment B and then A), so each subject can act as his or her own control, assuming that there are no carryover or seasonal effects. In a factorial design, two (and occasionally more) interventions, each with their own control, are evaluated simultaneously in one study. For example, one group tests treatment A, another tests treatment B, a third group tests A and B combined, and the control group tests neither A nor B. Such designs are already used extensively in cancer and cardiovascular studies and are likely to be needed increasingly in other conditions with multiple therapeutic options. Although they are an efficient method to test therapies in combination, achieving two comparisons for little more than the price of one, interactions between the interventions can complicate analysis of the outcomes and their interpretation. Bias in clinical trials is reduced by the use of random allocation and blinding. Randomization increases the likelihood (but does not ensure) that the groups will be well matched except for the intervention, distributing potential confounders both known and unknown between the intervention and control groups. Stratified randomization can be used to ensure that particular groups (e.g., the very old) are evenly distributed. Cluster randomization designs randomize groups of individuals (e.g., all those in a ward or nursing home) rather than individuals themselves and are increasingly common in health services research. Blinding means that the subject or investigator (single-blind) or both (double-blind) do not know to which group the subject is assigned. This prevents people from being treated differently in any way other than the intervention itself and helps ensure that outcome assessments are unbiased. National regulatory authorities, such as the Food and Drug Administration (FDA) and European Medicines Agency (EMEA), require positive outcomes from RCTs before a drug or medical device is given marketing approval for patient use. They will have been preceded by extensive preclinical in vitro (laboratory) and in vivo (animal) testing that when appropriate, may include studies with nonhuman primate models of aging or transgenic animal models of disease. Clinical trials then progress through an orderly series of steps, commonly classified into phases I to IV. Recently the concept of preliminary phase 0 trials has also been introduced to describe exploratory, first in human studies using single subtherapeutic (microdoses) of the study drug or agent, designed to confirm that the drug broadly behaves in humans as predicted from preclinical testing. In phase I trials, the study drug or agent is tested in a small group of subjects (20 to 80) in single ascending dose (SAD) and multiple ascending dose (MAD) studies to assess a safe dosage range, the best method of administration, and tolerance and safety (pharmacovigilance). Changes in the pharmacokinetics and pharmacodynamics of many drugs in older people, especially the frail, may significantly affect the choice of dose and dosing frequency for clinical use. A phase I trial usually recruits healthy young adults, so care must be taken when extrapolating results to older patients. When the study indication is common in older people, phase 1 trials may recruit older healthy volunteers or patients with the relevant condition—for example, as in initial studies of immunotherapy for Alzheimer disease. In phase II trials, the study drug or agent is given to a larger group of subjects (100 to 300), generally patients with the study indication, to assess safety and dosing requirements further (phase IIA) and to undertake preliminary studies of efficacy (phase IIB). Usually, these proof of concept studies recruit a homogenous group of younger subjects to maximize the chances of success and minimize adverse events related to altered pharmacokinetics and pharmacodynamics, comorbid conditions, and drug interactions more characteristic of older patients. However, there have been calls for regulatory authorities to require phase II studies of new agents to be carried out in individuals aged 70 years and older.25 In phase III trials, the efficacy and safety of the study drug or agent is evaluated in RCTs; usually, two positive trials are required to gain approval from regulatory authorities. These require the recruitment of up to several thousand patients from multiple centers and can last for several years, depending on the study indication. It is at this phase that arbitrary exclusion criteria based on chronologic age is especially difficult to justify. Randomization stratified by age and predetermined subgroup analysis will allow any issues specific to older patients to become apparent. Phase IV (postmarketing) trials are designed to provide additional information about benefits and risks of treatment in long-term use in clinical practice. Serious adverse effects identified at this late stage in older patients have resulted in withdrawal or restricted use of several prominent drugs. The carefully controlled nature of RCTs may themselves mean that they have limited generalizability, because subjects are often a very well-defined, highly selected group. Extensive lists of inclusion and exclusion criteria may exclude those with other comorbidities or who are taking other medications, and the resulting trial population might bear little resemblance to patients normally presenting in the clinic. For example, a minority of hospitalized older patients with heart failure fit the profile of populations of clinical trials,26 with the result of unintended harm to patients when trial results were applied in clinical practice.26–28 Certainly, perceived gains from narrow eligibility criteria (e.g., smaller, shorter, safer, less expensive trials) are often outweighed by the loss in generalizability and clinical applicability of the results and by less opportunity to test preplanned subgroup hypotheses (including any effect of age).29 Pragmatic clinical trials tend to take all comers and best reflect the effectiveness rather than merely the efficacy of an intervention. Older people, especially the frail and very old, are too often excluded from RCTs, usually inappropriately and without justification.30,31 This results in an inadequate evidence base to guide practice, so clinicians are left having to extrapolate trial findings to older patients who often carry the greatest burden of disease, but in whom available treatments have not been studied. The EMEA has stated that “there is no good basis for exclusion on basis of advanced age alone. … unless there is reason to believe that this will endanger the patient.”5 Studies have shown broad agreement among a range of health professionals that exclusion from clinical trials on age grounds alone is unjustified (87%), and that underrepresentation of older people in trials causes difficulties for prescribers (79%) and patients (73%).32 Fortunately, over time, there does seem to be a slow shift from exclusion based on age toward more justified exclusion based on failing organ function.33 Nevertheless, a review of eligibility criteria of RCTs published in high-impact medical journals from 1994 to 2006 found that after inability to consent, age was the second most common exclusion criterion, with 38% of trials excluding those older than 65 years.34 Age bias can be still be seen in clinical trials of the most common conditions of older people, including cancer,35,36 cardiovascular disease,37,38 Parkinson disease,39 surgery,40 type 2 diabetes,41 osteoarthritis42 and urinary incontinence.43 In preauthorization phase II and III trials of recently approved medicines, upper age limits were applied in 30.7% of the trials, and a very small proportion of participants were aged 75 years and older, even for diseases characteristically associated with aging (e.g., venous thromboembolism, osteoporosis, atrial fibrillation).44 Discrimination seems to be more common in Europe than in the United States and in drug trials sponsored by public institutions rather than private institutions.33 However, a review of RCTs specifically involving very old subjects concluded that their methodologic quality did not differ from comparable trials in the general population.45 Reasons given for excluding older subjects from research include concerns about gaining consent, protocol eligibility criteria with restrictions on comorbidities and concomitant medications, worries about poor compliance and high attrition, and fears of an unacceptable level of adverse events limiting the ability to identify an effect of treatment. If relatively large numbers of very old patients need to be screened to enroll an eligible subject, then it may be considered to be inefficient in terms of money and time to try and recruit them. Many of these concerns, however, are unfounded or can be easily overcome.46,47 A systematic review48 examining participation of older patients in phase III publicly funded RCTs in cancer between 1955 and 2000 found that in those trials with sufficient numbers of older enrollees, survival, event-free survival, and treatment-related mortality outcomes were similar to outcomes reported in the remainder of the studies. The authors concluded that the similarity in these two groups show that the enrollment of older adults in experimental RCTs is not associated with increased harm.
Methodologic Challenges of Research in Older People
Introduction
Study Designs
Qualitative Methodologies
Ecologic Studies
Cross-Sectional Studies
Case-Control Studies
Cohort Studies
Clinical Trials
Exclusion of Older People from Research
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Methodologic Challenges of Research in Older People
6






