Metastatic Epidural Spinal Cord Compression
Spinal metastases can be classified according to anatomic location and are considered to be either intradural (intramedullary or extramedullary) or extradural (epidural). Extradural lesions account for up to 95% of spinal lesions and may be divided into pure epidural lesions and those originating from the vertebra and subsequently impinging on the thecal sac. Pure epidural lesions are relatively rare. Metastatic epidural spinal cord compression (MESCC) is, thus, caused by a tumor extrinsic to the dura that compresses the spinal cord or cauda equina nerve roots. After the lungs and liver, bone (including the spine) is the system most often involved by metastases. MESCC is the most common neurological complication of cancer after brain metastases. MESCC usually occurs in patients with disseminated disease but may present as an isolated finding in patients not diagnosed with cancer. The progression of nearly all types of malignancies can be complicated by the occurrence of spinal cord compression. Different incidence rates are reported with different tumor types, but both breast and lung cancer each account for 15% to 20% of cases of MESCC1, 2 and prostate cancer approximately 14%.3 If left untreated, virtually 100% of patients will become paraplegic. Although most patients with MESCC have limited survival, up to one third will survive beyond 1 year.4
MECHANISM
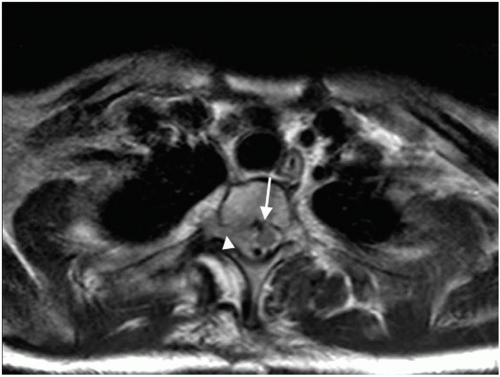
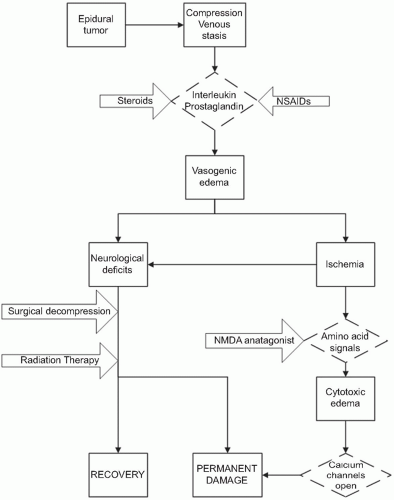
Most epidural spinal cord compression comes from a solid tumor metastasis to the vertebral body, which spreads posteriorly to the epidural space (observed in 85% to 90% of cases), and presents as an osteolytic bony lesion in 70% of patients resulting in anterior compression of the spinal cord (Fig. 11.1). Other tumors such as lymphoma, paragangliomas, and neuroblastomas, which invade the epidural space through the intervertebral foramina, account for 10% to 15% of cases. The frequency of metastasis appears to correlate with the volume of bone in that region of the spine, which is presumably related to blood flow and blood-borne metastasis. As such, the thoracic spine is most likely to have a metastatic lesion, owing to its 12 vertebral bodies; the lumbosacral spine is the next most likely to have involvement, owing to the large size of the vertebral bodies; and the small cervical vertebral bodies are the least likely to be affected. The most significant damage caused by MESCC appears to be vascular in nature. The epidural tumor causes epidural venous plexus compression, which leads to spinal cord edema (Fig. 11.2). The increased vascular permeability and edema lead to increased pressure on the small arterioles. Capillary blood flow diminishes as the disease progresses, leading to white matter ischemia. Prolonged ischemia eventually results in infarction and permanent cord damage.5, 6 The early mechanism of injury is vasogenic edema of white matter with direct involvement of cytokines, inflammatory mediators, and neurotransmitters.7 Production of vascular endothelial growth factor (VEGF) is associated with spinal-cord hypoxia and has been implicated as a potential mechanism of damage after spinal-cord injury.8 The beneficial effects of dexamethasone in the central nervous system (CNS) are at least partly mediated by its downregulation of VEGF expression.9 In the later stages of MESCC, vasogenic edema is replaced by ischemic-hypoxic neuronal injury and by onset of cytotoxic edema, a transition associated with the glutamate system that is characterized by release of presynaptic glutamate, influx of calcium through NMDAlinked ion channels, excitotoxic neuronal injury, and neuronal disintegration.10 Noncompetitive NMDA antagonists have been shown to delay the onset of paraplegia after onset of MESCC7 (Fig.11.3).
The segment of spine involved (approximately 10% cervical, 70% thoracic, 20% lumbosacral) reflects the number and volume of vertebral bodies in each anatomic segment.11, 12, 13 MESCC at one site is often accompanied by spinal involvement elsewhere, particularly in the case of widely disseminated breast or prostate cancer or myeloma. Reported frequencies of second asymptomatic epidural metastases range from 8% to 37%. Multiple sites of metastatic epidural spinal cord compression occur in 17% to 30% of all patients.14 This is particularly common in breast cancer and is uncommon in lung cancer.12
Severe, local back pain that gradually increases in intensity over time is the earliest and most common symptom (Fig 11.4). In general, pain occurs an average of 7 weeks before other neurological deficits. As the bone marrow does not contain pain receptors, discomfort usually occurs only when the enlarging mass invades the periosteum, paravertebral soft tissues, or nerves. Pain may also be caused by the mass effect of the spinal cord compression itself, spinal instability, pathological fracture, and the inflammatory and nociceptor stimulating substances that malignant cells secrete. Progressive pain practically always occurs in patients with MESCC. Weakness is the second most common symptom, which develops in approximately 80% of patients. As the thoracic cord is the most common site of epidural metastases, weakness usually involves the lower limbs, causing gait disorders. Gait difficulties may also be caused by sensory ataxia, presumably from posterior column compression.
Certain spinal tracts appear to be more vulnerable to compression than others.15 The corticospinal tracts and posterior columns are particularly vulnerable, the spinothalamic tracts and descending autonomic fibers less so. As a result, weakness, spasticity, and reflex hyperactivity tend to be the earliest signs of spinal cord compression, with paresthesias and vibratory and position sense loss occurring soon thereafter. Loss of pain and temperature sensation and of bladder and bowel function usually occurs late in the course of spinal cord compression. The spinocerebellar pathways are also sensitive to compression, and at times ataxia may be the only sign of spinal cord compression.
Certain spinal tracts appear to be more vulnerable to compression than others.15 The corticospinal tracts and posterior columns are particularly vulnerable, the spinothalamic tracts and descending autonomic fibers less so. As a result, weakness, spasticity, and reflex hyperactivity tend to be the earliest signs of spinal cord compression, with paresthesias and vibratory and position sense loss occurring soon thereafter. Loss of pain and temperature sensation and of bladder and bowel function usually occurs late in the course of spinal cord compression. The spinocerebellar pathways are also sensitive to compression, and at times ataxia may be the only sign of spinal cord compression.
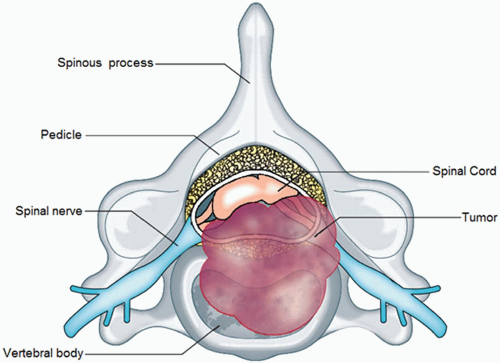 FIGURE 11.1 Tumor in the vertebral body. (From Cole JS and Patchell RA. Metastatic epidural spinal cord compression. Lancet Neurol 2008 7(5):459-466. Copyright 2008, with permission from Elsevier.) |
PATTERN OF PAIN
Four patterns of pain have been associated with epidural metastases. The majority of patients have local pain over the involved vertebral body, which results from involvement of the vertebral periosteum, is dull and exacerbated by recumbency. The worsening of pain on recumbency is the most distinctive feature of the pain of epidural spinal cord compression. Many patients with cord compression find they must sleep in a sitting position. Even if pain is absent in the lying position, turning over in bed or rising from a lying position may be particularly painful.
Radicular pain from compressed or damaged nerve roots is usually unilateral in the cervical and lumbosacral regions and may be bilateral in the thorax, where patients often describe it as a tight band across the chest or abdomen. The pain is experienced in the overlying spine, deep in certain muscles supplied by the compressed root, and in the cutaneous distribution of the injured root. The pain is usually least severe when the patient is in a position that minimizes compression of the root and most severe in positions that compress or stretch the root. Pain
is also increased by increasing intraspinal pressure, eg, coughing, sneezing, and straining. Radicular pain is present in 90% of patients with lumbosacral, 79% with cervical and 55% with thoracic metastatic spinal cord compression.11
is also increased by increasing intraspinal pressure, eg, coughing, sneezing, and straining. Radicular pain is present in 90% of patients with lumbosacral, 79% with cervical and 55% with thoracic metastatic spinal cord compression.11
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree