Although the conventional paradigm for treating metastatic melanoma relies on systemic therapies, a surgical approach should be strongly considered in selected patients. A surgical approach may not be appropriate for all patients, but it can offer a rapid clearance of disease without the toxicity of systemic therapy. Patient selection is of paramount importance for surgery to be effective. The rationale for surgical intervention in the management of metastatic melanoma, selection factors to be considered, published results, and future directions are discussed in this article.
Patients diagnosed with stage IV melanoma are known to have a poor prognosis, with a median survival of only 11 to 12 months, because there are no consistently effective systemic therapies available at present. The reference standard of chemotherapy, dacarbazine, is associated with response rates of only 10% to 15%, very few of which are durable. Even the more aggressive approved therapy, interleukin-2, is associated with a 5-year survival rate of less than 10%. Although there are several promising agents in development, such as targeted therapies and novel immunotherapy approaches, durable responses are still rare, and survival differences are measured in weeks to months instead of months to years. It seems that achieving a complete response is the only means of prolonging survival in these patients, a feat that is rarely possible with even the most aggressive systemic therapy. Because of the dismal prognosis in most patients with distant metastases, surgical referral was rarely initiated in the past. However, it is increasingly recognized that long-term survival can be achieved with surgical intervention in carefully selected patients. The rationale for surgical intervention in the management of metastatic melanoma, selection factors to be considered, published results, and future directions are discussed in this article.
Rationale for surgical management
Trials of systemic therapy in melanoma have consistently demonstrated that a complete response is required for a durable survival benefit. However, response is accomplished in only a small percentage of treated patients, even with the administration of the most effective available therapies such as interleukin-2, which achieves a complete response in only 6% of patients. Surgical metastasectomy has the unique potential to remove all tumors with a short recovery period instead of subjecting a patient to months of systemic therapy, itself associated with potential significant morbidity and occasional mortality. The importance of a complete response to systemic therapy applies to a surgical approach as well; surgery is most effective when a complete resection of all tumors is possible. However, surgery may be indicated for patients with sites of disease that are causing significant morbidity, even though a surgical approach would not render the patient disease-free. In this setting, complete resection of the symptomatic site may have the goal of palliation and improving the quality of life, while at the same time improving performance status and making the patient a better candidate for novel systemic approaches.
Although randomized prospective trials are obviously the optimal means of assessing the efficacy of surgery compared with that of other therapies, the small size and heterogeneity of this population limits true prospective analysis of survival. Some of the most convincing data supporting surgical management were obtained from an adjuvant vaccine trial, the Malignant Melanoma Active Immunotherapy Trial (MMAIT), a phase 3, randomized, double-blind trial of the polyvalent adjuvant vaccine, onamelatucel-L (Canvaxin), based on some promising retrospective matched data. To be eligible, patients were required to undergo a resection of all distant metastatic disease. This resection was followed by postoperative imaging confirming no evidence of disease before being randomized to receive either Canvaxin with BCG or BCG with a placebo. Although the vaccine group had similar survival rates to the placebo group, the 5-year overall survival rate for both groups was surprisingly high (40%–45%). The survival rate was much higher than previously reported, supporting that long-term survival was possible in this carefully selected stage IV population. Similar data were reported from the Southwest Oncology Group (SWOG) trial S9430, in which patients who underwent complete surgical resection for metastatic melanoma were prospectively followed up. Unlike the MMAIT trial, which included only patients who underwent a staging evaluation after complete resection and were found to still be without disease, the data from the SWOG prospective registry included all patients who underwent complete surgical resection. The SWOG registry included 62 patients from 18 institutions, with an overall median survival of 21 months and a 4-year overall survival of 29%. The favorable results from these 2 trial settings support the use of surgery in a selected population.
Factors for consideration
Surgery is not appropriate for all patients with metastatic spread of their melanoma. Patient selection is of paramount importance when discussing surgical management. In a retrospective review of 4426 patients with stage IV melanoma treated at the John Wayne Cancer Institute (JWCI), only 35% underwent surgical resection. These patients had a 5-year overall survival of 23% compared with 6% in those who were not surgical candidates ( P <.001). Surgery with the intent of providing long-term survival should be considered only when all sites of disease can be completely resected. Incomplete resection, similar to a partial response to systemic therapy, does not increase survival in these patients and should be considered only for palliation in symptomatic patients.
Radiographic Assessment
Determining the extent of disease is important. First, other primary cancers must be considered when a radiographic abnormality is seen in a patient with melanoma, particularly in the setting of an isolated lesion in the lung or the kidney. Further imaging, blood tests, and biopsy are sometimes necessary to confirm true metastatic disease. Second, the actual imaging modality is dictated by the site of known disease. For instance, an abnormality seen on chest radiograph should be further evaluated with computed tomographic (CT) imaging. Finally, a thorough evaluation must be performed to exclude or identify other sites of disease. This evaluation should include an assessment of the brain (usually with magnetic resonance imaging [MRI]), thoracic and abdominal cavities with CT or CT/positron emission tomography (PET) imaging, and a head-to-toe physical examination for the presence of skin, soft tissue, or nodal metastases. PET/CT is increasingly used in melanoma to evaluate for metastatic disease. Sensitivity rates of more than 90% and specificity rates similar to conventional CT have been reported, with the added advantage of being able to screen extremities and nodal basins for occult disease. This latter information may have a significant effect on the decision to proceed with surgical intervention; the identification of unexpected widespread disease excludes patients from surgical treatment, but the identification of a few sites of additional surgically resectable disease may expand the extent of surgery and more effectively render the patient disease-free.
Patient Factors
Unlike systemic therapy, surgery theoretically provides a means of rapidly rendering the patient disease-free, with low morbidity and a short recovery period. One key is that the patient can tolerate the proposed operation. Patients should expect to recover completely from thoracotomy or laparotomy within 4 to 8 weeks. For all operations, physicians must evaluate the overall health of the patient and their ability to tolerate the stress of surgery. In addition, certain site-specific factors should also be considered, such as pulmonary function tests, if a thoracotomy is planned. In the case of skin and soft tissue resections, the surgeon should be mindful of the functional deficits inherent in the planned operation and whether the patients and their support systems will be able to adapt.
Biology of Disease
Because the disease course of melanoma is variable, many groups have tried to identify factors associated with increased survival. Certainly, there have been strides in the last several years recognizing the effect of disease biology on prognosis.
Petersen and colleagues identified risk factors for poor prognosis in their study of 1720 patients with pulmonary metastases, including a nodular primary lesion, more than 1 pulmonary nodule, a disease-free interval of less than 5 years, or presence of extrathoracic metastases (synchronous or metachronous). After grouping the subjects by the number of risk factors, they found significant differences in median survival between those with no risk factors (14 months), 1 factor (12 months), and at least 3 factors (5 months). Although all the available literature is based on retrospective data of heterogenous populations, disease characteristics suggesting a more slowly growing tumor, such as a low burden of disease, solitary site of metastasis, and prolonged disease-free interval, are consistently shown to be important in outcomes despite the site of the metastases. In an effort to quantitate this rate of growth, tumor doubling time has even been suggested as a criterion for determining whether patients should be offered pulmonary metastasectomy.
Pattern of spread is another feature that may play a role in determining prognosis. In one study of 1500 patients with distant melanoma metastases, features that predicted a better survival regardless of treatment included the original site of metastasis and the stage of disease before metastasis. The difference in prognosis based on the anatomic site of metastasis is well established and is even reflected in the staging system as discussed later. Other groups have also shown that patients who progress from stage I or stage II to stage IV disease fare better than those who have regional nodal spread, or stage III disease, before the discovery of distant metastases. Not surprisingly, the disease-free interval between stage I or stage II disease and the development of metastases is longer (44 months) than after stage III disease (13.5 months), which makes it unclear if it is truly the presence of nodal disease that portends the poor outcome or rather the shortened disease-free interval. Regardless, both are evidence of an aggressively spreading tumor.
Factors for consideration
Surgery is not appropriate for all patients with metastatic spread of their melanoma. Patient selection is of paramount importance when discussing surgical management. In a retrospective review of 4426 patients with stage IV melanoma treated at the John Wayne Cancer Institute (JWCI), only 35% underwent surgical resection. These patients had a 5-year overall survival of 23% compared with 6% in those who were not surgical candidates ( P <.001). Surgery with the intent of providing long-term survival should be considered only when all sites of disease can be completely resected. Incomplete resection, similar to a partial response to systemic therapy, does not increase survival in these patients and should be considered only for palliation in symptomatic patients.
Radiographic Assessment
Determining the extent of disease is important. First, other primary cancers must be considered when a radiographic abnormality is seen in a patient with melanoma, particularly in the setting of an isolated lesion in the lung or the kidney. Further imaging, blood tests, and biopsy are sometimes necessary to confirm true metastatic disease. Second, the actual imaging modality is dictated by the site of known disease. For instance, an abnormality seen on chest radiograph should be further evaluated with computed tomographic (CT) imaging. Finally, a thorough evaluation must be performed to exclude or identify other sites of disease. This evaluation should include an assessment of the brain (usually with magnetic resonance imaging [MRI]), thoracic and abdominal cavities with CT or CT/positron emission tomography (PET) imaging, and a head-to-toe physical examination for the presence of skin, soft tissue, or nodal metastases. PET/CT is increasingly used in melanoma to evaluate for metastatic disease. Sensitivity rates of more than 90% and specificity rates similar to conventional CT have been reported, with the added advantage of being able to screen extremities and nodal basins for occult disease. This latter information may have a significant effect on the decision to proceed with surgical intervention; the identification of unexpected widespread disease excludes patients from surgical treatment, but the identification of a few sites of additional surgically resectable disease may expand the extent of surgery and more effectively render the patient disease-free.
Patient Factors
Unlike systemic therapy, surgery theoretically provides a means of rapidly rendering the patient disease-free, with low morbidity and a short recovery period. One key is that the patient can tolerate the proposed operation. Patients should expect to recover completely from thoracotomy or laparotomy within 4 to 8 weeks. For all operations, physicians must evaluate the overall health of the patient and their ability to tolerate the stress of surgery. In addition, certain site-specific factors should also be considered, such as pulmonary function tests, if a thoracotomy is planned. In the case of skin and soft tissue resections, the surgeon should be mindful of the functional deficits inherent in the planned operation and whether the patients and their support systems will be able to adapt.
Biology of Disease
Because the disease course of melanoma is variable, many groups have tried to identify factors associated with increased survival. Certainly, there have been strides in the last several years recognizing the effect of disease biology on prognosis.
Petersen and colleagues identified risk factors for poor prognosis in their study of 1720 patients with pulmonary metastases, including a nodular primary lesion, more than 1 pulmonary nodule, a disease-free interval of less than 5 years, or presence of extrathoracic metastases (synchronous or metachronous). After grouping the subjects by the number of risk factors, they found significant differences in median survival between those with no risk factors (14 months), 1 factor (12 months), and at least 3 factors (5 months). Although all the available literature is based on retrospective data of heterogenous populations, disease characteristics suggesting a more slowly growing tumor, such as a low burden of disease, solitary site of metastasis, and prolonged disease-free interval, are consistently shown to be important in outcomes despite the site of the metastases. In an effort to quantitate this rate of growth, tumor doubling time has even been suggested as a criterion for determining whether patients should be offered pulmonary metastasectomy.
Pattern of spread is another feature that may play a role in determining prognosis. In one study of 1500 patients with distant melanoma metastases, features that predicted a better survival regardless of treatment included the original site of metastasis and the stage of disease before metastasis. The difference in prognosis based on the anatomic site of metastasis is well established and is even reflected in the staging system as discussed later. Other groups have also shown that patients who progress from stage I or stage II to stage IV disease fare better than those who have regional nodal spread, or stage III disease, before the discovery of distant metastases. Not surprisingly, the disease-free interval between stage I or stage II disease and the development of metastases is longer (44 months) than after stage III disease (13.5 months), which makes it unclear if it is truly the presence of nodal disease that portends the poor outcome or rather the shortened disease-free interval. Regardless, both are evidence of an aggressively spreading tumor.
Prognosis and outcomes according to anatomic site
The anatomic site of distant metastases clearly affects prognosis in melanoma. This finding is reflected in the American Joint Committee on Cancer (AJCC) staging system, which added subcategories for stage IV disease in 2001 and further corroborated this approach in the most recent AJCC dataset published in 2009. Unlike most cancers in which patients are classified as either M0 if they have no known metastases or M1 if they have distant disease at any site, melanoma has 3 subgroups of M1 disease. M1a designates skin, soft tissue, or distant lymph node disease only; M1b represents pulmonary metastases; and M1c incorporates any visceral or brain sites or elevated lactate dehydrogenase (LDH) level (regardless of anatomic site of disease). The survival differences among these groups have been shown repeatedly, including analysis for the current seventh edition of the melanoma staging system, which was based on data from 7972 patients with stage IV melanoma from 17 institutions.
Skin and Soft Tissue Metastasis
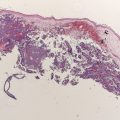
Distant skin and soft tissue metastasis represent about 40% of melanoma metastasis. As reflected in the staging system, isolated skin, soft tissue, or distant nodal metastases, or M1a disease, has the most favorable prognosis of patients with stage IV melanoma, with median survival of 18 months in all patients and 1-year survival rates of 62%. However, patients presenting with solitary dermal or subcutaneous metastasis that is completely resected can have survival rates of more than 80% at 8 years, although this rate represented only 11 patients of the 1800 patients with stage IV melanoma treated at that institution.
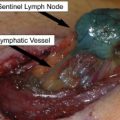
There are retrospective reports describing outcomes for patients with completely resected skin, soft tissue, or nodal disease. In a report from JWCI, 260 patients with completely resected disease had a median survival of 35 months and 5-year overall survival of 25%. In this study, patients with tumors confined to the skin or soft tissue instead of nodal metastases had a better prognosis, with a median survival of 48 months. This experience is similar to that at Memorial Sloan-Kettering Cancer Center (MSKCC), where 23 patients who had undergone previous lymphadenectomy for stage III disease underwent complete resections for isolated distant skin and soft tissue metastasis, with a median survival of 29 months and 5-year overall survival of 22%. The differences in survival observed between these 2 groups likely reflect slightly different underlying populations. The JWCI study included all patients with distant metastases, whereas the MSKCC group focused solely on patients who had a history of nodal disease. Other factors that predict a favorable outcome include skin or soft tissue location (instead of distant nodal spread), fewer lesions, prolonged disease-free interval, and small burden of disease.
The ability to aggressively resect distant skin, soft tissue, and nodal metastases has increased with the evolution of reconstructive options. In the case of bulky disease, the surgeon may need to consult with thoracic surgeons to aid with chest wall resections and/or plastic surgeons to provide tissue coverage. A variety of reconstructive options are reasonable, including skin grafts, rotational flaps, and free tissue transfer. Staged procedures are sometimes required for optimal results and definitive margin assessment ( Table 1 ).