Fig. 7.1
Relationship between mid-thigh low-density lean tissue and insulin sensitivity, M/I (r = −0.33, P = 0.05) in African American and Caucasian women
In the Kronos Early Estrogen Prevention Study, healthy early postmenopausal women (n = 650) underwent computed tomography (CT) imaging to study cardiac and intra-hepatic fat and their relationship to metabolic risk factors [19]. Increased epicardial adipose tissue was associated with high LDL-cholesterol, triglycerides, glucose, insulin, hs-C-reactive protein (CRP), and low HDL levels. Likewise, higher pericardial adipose tissue was associated with increased triglycerides, insulin, hs-CRP, and low HDL-cholesterol. Results of these associations of cardiac fat, in general, persisted after adjustment for total obesity by BMI and abdominal fat (waist circumference). In addition, hepatic fat was associated with a worse profile of the above risk factors in this large group of postmenopausal women [19]. In another study, uric acid, BMI, waist circumference, alanine aminotransferase, triglycerides levels, and HDL-cholesterol were associated with insulin resistance measured by Homeostasis model assessment (HOMA) in postmenopausal women without diabetes [20]. In summary, there is evidence in postmenopausal women that subcutaneous abdominal fat and ectopic fat (visceral fat, thigh intramuscular fat, and hepatic fat) are associated with increased disease risk.
In a comprehensive longitudinal examination of changes in body composition during menopause, Lovejoy et al. [21] found that women who became postmenopausal by the fourth year of the study had gained weight and body fat. In addition, only women who became postmenopausal had an increase in visceral fat, whereas all women gained subcutaneous abdominal fat over time. The group also reported that not only did energy expenditure by accelerometry decline as the women aged but 24-h energy expenditure and substrate oxidation measured with a whole-room calorimeter decreased over time, with greater decreases in the women who became postmenopausal [21]. This study was the first longitudinal investigation to substantiate the cross-sectional observations of increases in abdominal fat in women during the perimenopausal years. It is thought that fat distribution changes may be influenced by sex hormone concentrations.
Hormonal Status
Several reports have examined hormones or metabolites such as insulin, sex hormone-binding globulin (SHBG), testosterone, leptin, and adiponectin to investigate hormonal status in postmenopausal women. A higher fasting insulin is associated with a worse lipoprotein lipid profile (increased triglyceride and reduced HDL-cholesterol) in postmenopausal women [22]. Insulin was also associated with apolipoprotein A–I after adjustment for abdominal adiposity, estrone, and SHBG [22]. When body fat is similar between perimenopausal and postmenopausal women, fasting insulin is not different between groups [23]. Fasting insulin increased with an associated weight gain in women studied over a 3-year period of menopause [24].
SHBG is a serum glycoprotein that binds testosterone with high affinity and estrogen with lower affinity and is considered an indirect marker of androgenicity. Serum SHBG is negatively associated with obesity [25] and visceral fat [26]. SHBG is also associated with insulin resistance and glucose tolerance in postmenopausal women [27, 28] and is an independent marker of risk for type 2 diabetes [29]. In over 750 postmenopausal women who were lean to obese (BMI range 15–53 kg/m2), HOMA-IR, BMI, and diastolic blood pressure were inversely related to serum SHBG and combined, explained approximately 34 % of the variation of SHBG [30]. Furthermore, there was a positive relationship between serum SHBG and serum total testosterone; however, the relationship between SHBG and insulin resistance was independent of circulating testosterone [30], suggesting that androgens do not help explain this relationship. Others have shown that serum SHBG is negatively correlated with metabolic syndrome ( [31] E) and markers of inflammation including CRP [32–34] in women. Moreover, serum SHBG is inversely correlated with serum CRP concentrations even after adjustment for age, components of the metabolic syndrome, insulin resistance, LDL-cholesterol, serum sex hormones, estradiol, and total testosterone [33], suggesting that SHBG may be independently associated with inflammation in Asian postmenopausal women.
Leptin, a product of the OB gene in humans, has been studied in the context of obesity and glucose homeostasis given that the OB protein regulates body weight and fat deposition through alterations in appetite and metabolism [35, 36]. Leptin is highly correlated with percent fat, subcutaneous abdominal fat, and visceral fat in women [37]. Moreover, leptin is associated with fasting insulin even after controlling for body fat. We also found that plasma leptin levels changed little from basal during hyperglycemic (approximately 10 mmol/L0 or hyperinsulinemic-euglycemic (400–3,000 pmol/L) clamp studies in women athletes and controls [37]. In ~150 postmenopausal women, serum leptin levels were significantly higher in women with the metabolic syndrome than those without the metabolic syndrome even after adjustment for BMI, WHR, and visceral fat [38]. Leptin is also associated with BMI and total fat mass in Japanese postmenopausal women with knee osteoarthritis [39]. Menopausal status is a significant predictor of both leptin and adiponectin in premenopausal and postmenopausal Tunisian women [40]. In another cross-sectional study, plasma leptin was associated with BMI and percent body fat in physically active postmenopausal women between the ages of 50 and 85 [41].
Adiponectin, a peptide expressed specifically and abundantly in adipose tissue [42, 43], is considered an anti-inflammatory and insulin-sensitizing adipokine which is lower in obesity [44]. In approximately 150 women, adiponectin and leptin levels were measured to examine the relationships among those adipocytokines, total and central obesity, and insulin sensitivity across the adult age span in women [45]. Adiponectin was negatively associated with BMI, percent fat, waist circumference, visceral fat, subcutaneous abdominal fat, and leptin [45]. In healthy postmenopausal Korean women, adiponectin was significantly negatively correlated with waist circumference, high-density lipoprotein cholesterol, diastolic blood pressure, and insulin resistance by HOMA model [46]. In the Women’s Health Initiative Observational Study, adiponectin levels were associated with stroke risk factors including obesity and systolic blood pressure [47]. We also determined peripheral tissue sensitivity to exogenous insulin using the hyperinsulinemic-euglycemic clamp and showed that in a multivariate analysis, only insulin sensitivity or M was a significant independent predictor of adiponectin [45]. The mechanism by which adiponectin exerts its insulin-sensitizing effects is through a decrease in muscle and liver triglyceride content [48]. In skeletal muscle, adiponectin increases fatty acid oxidation [48, 49] by inactivating acetyl CoA carboxylase (ACC) and activating AMP-activated kinase [50], thereby regulating glucose metabolism.
Racial Disparities in Postmenopausal Women
Several studies from our group suggest that race may differentially affect body composition in the older female. Postmenopausal African American women have significantly more subcutaneous abdominal fat and greater waist circumference than Caucasian postmenopausal women [15, 51]. Mid-thigh low-density lean tissue (a marker of intramuscular fat) is almost 35 % higher in postmenopausal African American than Caucasian women [15]. Studies are conflicting with regard to racial differences in visceral fat. Caucasian postmenopausal women had approximately 22 % higher visceral fat area but similar subcutaneous fat area in one study [14] and no differences in visceral fat area in others [15, 51, 52]. Subcutaneous abdominal fat at L2–L3 was lower in African American than Caucasian postmenopausal women so that the ratio of visceral to subcutaneous fat was lower in African than in the Caucasian women [52].
We have also studied postmenopausal women who are overweight or obese and sedentary and made several metabolic comparisons between African American and Caucasian women. Postmenopausal African American women had similar circulating levels of SHBG even after adjustment for body fat and fasting insulin as Caucasian postmenopausal women [51]. Furthermore, levels of testosterone did not differ between groups [51]. The associations of body composition, insulin, and lipids with SHBG differ between African American and Caucasian obese postmenopausal women. In African American women, SHBG is not related to central obesity, insulin, or HDL-cholesterol, but SHBG is related to these factors in Caucasian women. These results suggest that after menopause, sex steroid metabolism may affect regional fat distribution, lipid and glucose metabolism differently in African American and Caucasian women [51, 53, 54]. Postmenopausal African American women have higher insulin levels [15, 51], higher insulin area under the curve [51] and higher leptin concentrations [51] than Caucasian postmenopausal women. Leptin concentration was 20 % lower in obese postmenopausal African American than Caucasian women matched for level of body fat [55]. In an early study, we reported that postmenopausal African American women had 60 % lower glucose uptake, assessed during a 240 pmol/m2 per minute hyperinsulinemic-euglycemic clamp due to a 98 % lower nonoxidative glucose disposal [15]. Insulin sensitivity was negatively correlated with mid-thigh low-density lean tissue, suggesting that increased fat deposition in the muscle in African American women decreases insulin sensitivity. Rates of basal and insulin-suppressed lipolysis in abdominal fat were higher in African American than Caucasian postmenopausal women [52]. The decline in sex steroids with menopause may contribute to this finding. More studies are needed to investigate the mechanisms that may contribute to the body composition and metabolic differences between African American and Caucasian postmenopausal women.
Exercise Training in the Aging Female
Exercise training has beneficial effects on cardiometabolic health and traditional cardiovascular risk factors including obesity, hypertension, dyslipidemia, and glucose intolerance. Studies in highly competitive athletes offer a unique perspective to compare to healthy but sedentary older women. In comparisons of athletes and sedentary women, we have reported that women athletes (swimmers, runners, triathletes) between 18 and 69 years of age have lower percent body fat, fat mass, visceral, and subcutaneous abdominal fat than normal BMI age-matched controls [56]. Of particular interest to this chapter, the postmenopausal athletes had half the amount of visceral fat than the older controls as well as similar total body fat and subcutaneous abdominal fat to the young athletes implying that the competitive nature and increased endurance training enabled the older athletes to maintain a reduced adiposity despite their age [56]. We also studied glucose metabolism in these older women athletes and reported β-cell sensitivity to glucose and peripheral tissue sensitivity to insulin was preserved in women athletes as a function of age [57]. The older sedentary women had a 70 % greater first-phase insulin response, a 103 % greater second-phase insulin response during hyperglycemia, and utilized significantly less glucose than the older athletes, indicating increased insulin sensitivity in older athletes [57]. HDL-cholesterol is greater and LDL-cholesterol is lower in women athletes than sedentary women [58]. Moreover, total and LDL-cholesterol increased with age even after adjustment for fitness (VO2max) and visceral fat suggesting that changes in lipoproteins are due to primary aging [58]. These studies highlight the benefits of high-intensity training in older competitive women athletes.
Exercise, Body Composition, and Cardiometabolic Outcomes
Improvements in physical fitness and changes in body weight are important to changes in body composition. In sedentary obese postmenopausal women who participated in a walking and weight loss program, a 10 % increase in VO2max results in a 20 % decrease in visceral fat, whereas a lack of change in VO2max decreases visceral fat by about half that amount (10 % decrease) [59]. In several of our studies of different groups of postmenopausal women, we have examined changes in body composition after weight loss alone, or weight loss combined with aerobic training over a 6-month period. We report that an 8–10 % decrease in body weight results in a 13–18 % decrease in visceral fat and a 12–17 % decrease in subcutaneous abdominal fat [60–63]. Weight loss and walking also reduced intramuscular fat by ~4–18 % in postmenopausal women [60–62, 64]. To study the effects of exercise intensity on visceral fat loss, Nicklas et al. [65] conducted a randomized clinical trial in overweight and obese postmenopausal women enrolling in either 20 weeks caloric restriction alone, calorie restriction plus moderate exercise, or calorie restriction plus vigorous exercise. All groups lost significant amounts of weight with similar losses in visceral fat and subcutaneous abdominal fat [65]. Changes in the metabolic parameters (decreases in triglycerides, LDL-cholesterol, fasting glucose and insulin, and areas under the curve) did not differ by group. In this same trial of caloric restriction versus caloric restriction + moderate intensity, versus caloric restriction + vigorous intensity exercise in obese postmenopausal women, pericardial fat decreased slightly more than 15 % among all groups without a significant difference between groups [66]. These studies all suggest that reduction in weight by a decrease in energy intake is critical to the loss of fat in the various depots.
We have shown that resistive training alone does not result in a loss of body weight or total body fat but total body FFM and thigh muscle area increase after resistive training in postmenopausal women [67]. Others have also shown gains in muscle mass after resistive training postmenopausal women [68, 69]. After 10 weeks of unilateral strength training, muscle volume increased without changes in intermuscular fat or mid-thigh subcutaneous fat in women aged 50–85 years of age [70]. The women over the age of 65 then completed a 12-week program of whole-body strength training and had significant increases in FFM [71]. Melnyk et al. [72] reported increased proximal, middle, and distal quadriceps muscle areas of the thigh after a 9-week one-legged strength training program in older (65–75 years) women, suggesting that the increases in muscle area occur across the entire thigh muscle. In contrast, one study has shown that 6 months of progressive whole-body resistive training did not change lean mass in older aged [65–74] year women [73]. Visceral fat has been reported to decrease after resistive training in older women [74]. On the contrary, visceral fat and subcutaneous abdominal fat did not change but total body FFM increased by ~1 kg in community-dwelling frail elderly (>78 years of age) women after a 3-month progressive resistance training program that followed an aerobic exercise training intervention [75]. In a randomized control trial, Kemmler et al. [76] showed that appendicular skeletal muscle mass and total lean body mass increased and abdominal and total body fat by DXA decreased after an 18-month high-intensity exercise (aerobic, balance, strength components) in 250 elderly community-dwelling women. Thus, in contrast to aerobic exercise that results in minimal changes in FFM, resistive training alone may increase total body and regional muscle mass but the population studied may be important with respect to body composition results.
Endogenous sex hormones were studied in overweight postmenopausal women with impaired glucose tolerance who underwent an intensive lifestyle modification program or metformin of the Diabetes Prevention Program [77]. SHBG levels increased and dehydro-epiandosterone (DHEA) decreased without changes in estradiol or testosterone in the lifestyle group. Furthermore, changes in SHBG and DHEA were associated with reductions in both fasting and 2-h glucose levels independent of changes in waist circumference and fasting insulin [77]. An 8-week cycle exercise program decreased fasting insulin and insulin-like growth factor (IGF)-1 levels and did not change IGF binding protein-3 in a small sample of overweight and obese postmenopausal women [78]. In sedentary postmenopausal women from Brazil, a 16-week resistive training program increased IGF-1 levels but total testosterone, estradiol, and cortisol remained unchanged [69].
Exercise and Energy Expenditure
Postmenopausal women had a reduction in daily physical activity energy expenditure on the days they participated in center-based moderate or vigorous exercise [79]. The authors suggest that postmenopausal women may compensate for the increased energy expended during exercise sessions by reducing their activity/energy levels outside the structured program [79]. This could have important implications for weight control and obesity in postmenopausal women, especially for women who are trying to maintain their current weight or prevent weight regain after weight loss.
Sedentary overweight and obese postmenopausal women who have higher levels of physical activity energy expenditure have better lipoprotein and lipid profiles and lower circulating inflammation than those women with lower activity levels [80]. In a post hoc analysis combining two cross-sectional studies in inactive postmenopausal women, Lavoie et al. [80] reported that the interaction of physical activity energy expenditure by doubly labeled water and diet quality by the Canadian Healthy Eating Index were associated with higher HDL-cholesterol, apoB, LDL-cholesterol/apoB ratio, and lower hs-CRP levels, suggesting that physical activity and dietary habits work synergistically to create a favorable cardiometabolic risk factor profile.
Exercise and Glucose Metabolism
Cross-sectional and longitudinal studies consistently demonstrate the benefits of exercise on glucose homeostasis. In a large population of elderly men and women, physical activity, by self-administered questionnaire decreased with increasing glucose intolerance and persisted after adjustment for age, BMI, waist–hip ratio, family history of diabetes, and smoking [81]. This was confirmed in a similar recent study where insulin sensitivity by a euglycemic clamp was not different in younger women athletes versus older endurance-trained athletes [82]. Since the normal-weight younger subjects have similar insulin sensitivity to the older subjects, it could suggest that the obesity and physical inactivity are more important in insulin resistance than aging per se [82]. Insulin sensitivity as estimated by the homeostatic metabolic assessment for insulin resistance (HOMA-IR) was determined in over 750 athletes representing 33 different sports [83]. Those athletes with the lowest HOMA-IR values were rowers and short-distance track athletes, whereas archery and field-throwing athletes had higher HOMA-IR values than the control group. Weight-lifting athletes may not confer the same metabolic benefits in terms of glucose metabolism as aerobically trained athletes.
Aerobic exercise training can improve glucose metabolism in overweight and obese postmenopausal women by reducing glucose and insulin levels during oral glucose tolerance tests and increasing glucose utilization during glucose clamps [61, 64]. The exercise effects may be long lasting in older women given that insulin sensitivity increased approximately 20 % even when glucose clamps are performed 72 h after the last training session [84]. When whole-body insulin resistance is estimated by HOMA-IR, it decreases with diet alone and exercise + diet but not exercise alone compared to control in overweight and obese postmenopausal women [85]. We recently conducted a large clinical trial of caloric restriction alone and combined with 6 months of aerobic training. Postmenopausal women underwent skeletal muscle biopsies prior to and during a hyperinsulinemic-euglycemic clamp both before and after the interventions to examine the mechanisms responsible for improved insulin sensitivity with these lifestyle interventions [62]. The effect of in vivo insulin to increase glycogen synthase (GS) fractional activity is strongly correlated to whole-body glucose utilization (Fig. 7.2). In addition, GS fractional activity was significantly lower in women with impaired glucose tolerance than women with normal glucose tolerance which likely contributes to the insulin resistance in women with impaired glucose tolerance. As a result of the interventions, the change in glucose utilization was associated with the change in insulin-stimulated GS fractional activity. In examining this further, we found that in women with impaired glucose tolerance, there is an enhanced insulin-stimulated GS activity following aerobic exercise + caloric restriction and the effect of insulin to increase GS total activity is greater after the combined intervention than caloric restriction alone [62]. Our results would suggest that adding aerobic exercise to caloric restriction improves insulin sensitivity in overweight and obese postmenopausal women at risk for diabetes and that changes in GS activity may be one contributing mechanism.
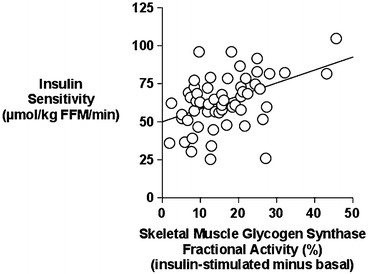

Fig. 7.2
Relationship between skeletal muscle glycogen synthase (GS) fractional activity (insulin-stimulated minus basal) and insulin sensitivity (M, glucose utilization) in postmenopausal women (r = 0.45, P < 0.005)
Resistive training has also been studied as a mode of exercise to improve glucose homeostasis in postmenopausal women. Resistive training does not change fasting plasma glucose [86] regardless of age or sex [87]. Yet, resistive training does improve glucose metabolism in women [86–88]. A 4-month resistive training program alone and resistive training plus weight loss increased insulin action and reduced hyperinsulinemia as assessed by hyperglycemic clamps in middle-aged postmenopausal women [88]. Furthermore, a 6-month resistive training program tended to improve insulin action in older postmenopausal insulin-resistant women, and the change in glucose utilization was a function of initial glucose utilization [86]. Even though there are a limited number of metabolic studies after resistive training in postmenopausal women, the current evidence would imply that this type of exercise is advantageous in the aging female.
Additional Benefits of Exercise Training
Physical activity is also important to women during and after menopause for physical and psychological reasons. The benefits of exercise training on increasing bone mineral density or reducing the loss of bone density with aging in older women are beyond the scope of this chapter. Readers are encouraged to peruse other chapters or reviews on this topic [89–91]. There are a few studies which report the importance of physical activity in enhancing quality of life among menopausal women [92], reducing frequency of hot flashes [93, 94], and controlling body weight or reducing a gain in body fat [95]. Since weight gain during menopause is associated with increases in total cholesterol, LDL-cholesterol, triglyceride levels, and blood pressure [24], weight reduction programs during and after menopause may be particularly important to reduce cardiovascular risk. Women (premenopausal, perimenopausal, postmenopausal) who have an increase in physical activity over an 8-year period have improved quality of life compared to women whose physical activity declined [96]. Quality of life deteriorated in more women who were in the menopausal transition than postmenopausal women [96]. This indicates that increased physical activity levels in this period may be critically important.
Summary and Future Directions in the Aging Female
It is clear that obesity remains a significant concern for the aging female. The cardiovascular risk factors that accompany obesity such as dyslipidemia, hypertension, and glucose abnormalities, warrant the continued emphasis on initiating and maintaining exercise programs in the aging female. New nontraditional types of exercise should be explored in healthy women and older women with disabilities. As well, more studies should focus on elderly postmenopausal women as less scientific evidence and information is available for this group. The cellular mechanisms that underlie metabolic improvements after exercise training in postmenopausal women and the interaction with sex steroids remain fruitful areas of investigation.
Acknowledgments
Dr. Ryan was supported by a VA Research Career Scientist Award, Veterans Affairs Merit Review Award, NIH grant R01-AG-030075, Mid-Atlantic NORC (DK072488), and the University of Maryland Claude D. Pepper Center (P30-AG-12583).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree