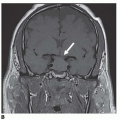
Atypical Femoral Fractures
There has been concern about atypical femoral fractures in association with bisphosphonate use. The American Society for Bone and Mineral Research (ASBMR) task force issued a statement [
4] regarding atypical subtrochanteric and diaphyseal fractures (see
Table 4.7). Based on review of the available data while a causal relationship between the use of bisphosphonates and atypical fractures has not been established, it was postulated that bisphosphonates can potentially contribute to factors increasing risk of these fractures. These include reduced angiogenesis, alteration in collagen cross-linking and maturation, increased advanced glycation
end products, reduced heterogeneity of bone mineralization, bone remodeling, and microdamage accumulation. This report [
4] included data from the Study of Osteoporotic Fractures (SOF), which was a prospective population-based US study of 9,704 Caucasian women over the age of 65, which showed an overall incidence of subtrochanteric fractures to be 3 per 10,000 patient-years (compared to 103 cases per 10,000 patient-years for overall incidence of hip fractures). Older age, lower total hip BMD, and history of falls were identified as predictors of subtrochanteric fractures; however, after multivariate analysis, only age remained a significant factor.
The task force also reviewed data from a large US Health Maintenance Organization (HMO), which included 15,000 total hip and femur fractures in women over the age of 45. Data from 600 possible atypical fractures were examined, 102 had features consistent with atypical fracture, and 97/102 patients had been treated with bisphosphonates. Preliminary estimates showed a progressive increase in risk of atypical fracture from 2 per 10,000 cases per year to 78 per 10,000 cases per year with 2 and 8 years of bisphosphonate treatment, respectively. While the incidence of atypical fractures was low and there was no control group to compare to, there was an increased risk of atypical femoral fractures with longer duration of bisphosphonate treatment.
Based on case reports and case series, 310 cases have been reported with 286 occurring in patients treated with bisphosphonates for osteoporosis and five patients treated for malignancy; there was no bisphosphonate use in the remaining cases. The duration of bisphosphonate therapy ranged from 1.3 to 17 years with mean duration of 7 years. Glucocorticoid use was identified in 76 of the cases. Other risk factors noted in some series have included proton pump inhibitors (PPI) use, comorbid conditions (i.e., RA or diabetes mellitus [DM]), age younger than 65 (which is in contrast to the SOF data), and vitamin D deficiency.
There is limited histologic data available in patients with atypical femoral fractures with majority of the data obtained from iliac crest biopsies and a small number of biopsies performed at or near the subtrochanteric fracture region. However, it is important to keep in mind that the biopsy results from the site of the fracture can be affected by the changes occurring in response to the fracture. In general, the biopsies have revealed low bone turnover and lack of double tetracycline labeling (in some cases, single label was present).
The results of bone markers have not been consistent. In many cases, the BTMs were not suppressed to the extent that would be anticipated based on biopsy results, but it is important to keep in mind that the markers may have been affected by the fracture itself. More data from biopsy results obtained from patients on bisphosphonate treatment with and without atypical femoral neck fractures as well as data regarding BTMs are necessary to shed light on changes present that set atypical fractures apart from other types of fractures.
Based on available data, the incidence of atypical femoral neck fractures is very low and the risk benefit profile favors using bisphosphonates for fracture prevention. However, it is important to note that the incidence of atypical femoral fractures may be underestimated secondary to lack of awareness and underreporting. It has been recommended to establish an international registry of patients with atypical fractures and conduct further research to identify the clinical and genetic risk factors that lead to higher risk of developing atypical femoral fractures [
46].
A secondary analysis of the FIT, FLEX, and HORIZON trials reviewed the incidence of subtrochanteric and diaphyseal femur fractures. In this study, femoral neck, subcapital, periprosthetic, pathologic, and high-energy fractures were excluded. Overall, this study identified 12 atypical fractures, a combined rate of 2.3 per 10,000 patients. The data showed that the risk of atypical fracture associated
with bisphosphonate use is very low. The relative hazard (RH) ratio compared to placebo was 1.03 (95% CI, 0.06-16.46) for the FIT (alendronate) trial, 1.33 (95% CI, 0.12-14.67) for the FLEX trial (continuation of FIT), and 1.5% (95% CI, 0.25-9.00) for the HORIZON (zoledronic acid) trial. It is important to note several limitations of this study including lack of radiographic information to assess the atypical features of the fractures, the small number of events (therefore low statistical power), exclusion of patients treated with confounding medications by design, and lack of long-term data [
47].
It is not clear which patients are at higher risk of atypical fractures with bisphosphonate use, but case reports have suggested that longer duration of bisphosphonate treatment (however, the reported ranges of bisphosphonate treatment has been between 1.3 and 17 years, with median duration of 7 years) and the concomitant use of corticosteroids, PPIs, and other antiresorptive treatments may be associated with higher risk of atypical fractures.
To evaluate fractures, conventional radiographs (anteroposterior and lateral views) can be sufficient. In equivocal cases or in patients with normal radiographic findings but high suspicion for an evolving fracture technetium bone scan, MRI or spiral CT may be helpful. For patients with atypical fracture, bisphosphonate treatment should be stopped. Orthopedic evaluation may be necessary. In case reports, teriparatide has shown promise in fracture healing and can be a consideration for treatment [
46].
The optimal duration of bisphosphonate treatment is not clear. The duration of treatment should be based on history of fractures, fracture risk, and BMD. Markers of bone turnover may aid in decision-making as well. Treatment with bisphosphonates for 5 years appears to be safe and provide antifracture benefit.
In the FLEX trial, there was a lower risk of clinical vertebral fracture in patients who remained on alendronate for 10 years compared to those who stopped alendronate after 5 years [
17]. Based on the risedronate data, treatment for 7 years did not lead to further fracture reduction compared to 3 and 5 years of treatment [
24]. According to the 2010 AACE guidelines, it is reasonable to give a “drug holiday” after 4 to 5 years of bisphosphonate therapy for patients with low or moderate risk of fracture and 10 years for high-risk patients [
48]. For high-risk patients, alternative agents, such as teriparatide, could be used during the drug holiday.
This is an evolving area that needs more data; specifically, it is not clear if a drug holiday will decrease the risk of atypical femoral fractures and the optimal duration of the drug discontinuation period. After discontinuing bisphosphonates, it is important to closely monitor clinical status, bone markers, and BMD. Resuming treatment can be considered if there is a fracture or a significant decline in BMD.
Raloxifene
Raloxifene (Evista) is a selective estrogen receptor modulator, with agonistic effects on bone. The major efficacy trial for raloxifene was the Multiple Outcomes of Raloxifene Evaluation (MORE) trial [
49]. The LS BMD increase over the 3-year study period was 2% to 3%, and vertebral fracture—reduction rates in women with and without preexisting fractures were 50% and 30%, respectively. No significant difference in nonvertebral and hip fracture reduction was observed. Efficacy of raloxifene was sustained through 4 years of treatment [
50]. A meta-analysis of seven trials comparing raloxifene and placebo showed a similar BMD increase at the LS and a 2% increase for the combined hips [
51].
The Continuing Outcomes Relevant to Evista (CORE) trial was a 4-year extension of the MORE trial. The placebo-treated group continued with placebo, and those previously treated with raloxifene (60 or 120 mg/d) received raloxifene 60 mg/d. The secondary end point of the study was new nonvertebral fractures. The risk of at least one new nonvertebral fracture was similar in the placebo (22.9%) and raloxifene (22.8%) groups, with an HR of 1.00 (Bonferroni-adjusted CI, 0.82, 1.21). Based on a subgroup analysis, 7 years after MORE randomization, the difference in the LS and femoral neck BMD with raloxifene were 1.7% (
p = 0.30) and 2.4% (
p = 0.045), respectively, compared to placebo. Compared to MORE baseline, 7 years of raloxifene treatment significantly increased LS (4.3% from baseline, 2.2% from placebo) and femoral neck BMD (1.9% from baseline, 3.0% from placebo). At all time points, femoral neck and spine BMD were significantly higher in the raloxifene group compared to MORE baseline. Some limitations of this study included differences in the populations studied. The women who enrolled in CORE were younger and had less severe osteoporosis compared to those who did not enroll. The placebo group had fewer prevalent fractures at MORE baseline compared to the raloxifene group. Of note, since CORE’s primary end point was cancer prevention, bone-active agents were permitted after the 3rd year of MORE study and a significantly higher percentage of women in the placebo group used bone-active treatment compared to the raloxifene group. Approximately 20% of CORE participants did not take the study drug. These differences may have affected the ability to detect a difference in fracture incidence between the groups. Also, BMD was assessed in a subgroup of patients and therefore may not have been representative of the entire group [
52].
This drug has other potential benefits, including reduction in breast cancer risk and improvement in lipids and markers of cardiovascular (CV) disease, but these are not discussed in this section.
Teriparatide (PTH 1-34)
Synthetic human PTH 1-34, or teriparatide (Forteo), is an anabolic agent that has been approved for treatment of postmenopausal and male osteoporosis. The landmark trial in postmenopausal women was the Fracture Prevention Trial (FPT). In this study, 1,637 postmenopausal women received placebo, 20 or 40 μg daily
subcutaneous injections of teriparatide for a mean of 21 months. In the groups treated with teriparatide, vertebral fractures decreased by 65% and 69%, respectively, and nonvertebral fractures were reduced by 53% and 54%. Mean increases in LS BMD of 9% and 13%, as well as 3% and 6% at the femoral neck, were seen. The most common side effects were nausea and headaches [
60].
Teriparatide is approved for only 2 years of use; therefore, it is of interest to see what happens to the bone mass of patients who discontinue the drug. Extensions of the FPT have looked at changes in BMD and fracture risk after discontinuation of teriparatide. One study found that 30 months after discontinuation of teriparatide, the hazard ratio for nonvertebral fragility fractures was still significantly lower than with placebo but only in the 40-μg group. BMD decreased over those months in both groups, except in those who received bisphosphonates for at least 2 years during the trial [
61]. Another study looked at vertebral BMD changes and fractures 1.5 years after discontinuing teriparatide. There continued to be a statistically significant increase in BMD and a decrease in fractures in those who had been taking teriparatide. Those who used bisphosphonates for at least 1 year continued to gain BMD, whereas those who did not lost BMD [
62].
A randomized study of 93 postmenopausal women with low BMD examined the effects of alendronate 10 mg/d, teriparatide 40 mg/d, or both over 30 months. The LS BMD increased more in the group treated with teriparatide compared to alendronate or the combination group. A similar pattern was observed for femoral neck BMD. Bone markers increased more in the teriparatide group compared to the alendronate or combination group. The results showed that alendronate reduced the anabolic effect of teriparatide [
63].
A randomized, double-blind trial compared teriparatide, 40 μg, with alendronate, 10 mg daily. By 3 months, and through the 14 months of the study, those in the teriparatide group experienced significantly greater increases in LS and hip BMD than with alendronate. The incidence of nonvertebral fractures was significantly lower in the teriparatide group compared to the alendronate group [
64].
The effects of teriparatide after administration of alendronate or raloxifene have been assessed. During the first 6 months, the prior raloxifene group had higher gains in BMD at the LS and the hip whereas the prior alendronate group did not. After the first 6 months, the rates of increase were similar in both groups. At 18 months, the raloxifene group had gained 10.2% in LS BMD, compared with 4.1% in the alendronate group (
p < 0.001) [
65].
Teriparatide also has been shown to increase bone mass by 13% in the LS and 2.9% in the femoral neck in men with idiopathic osteoporosis [
66]. A randomized trial of 83 men, with LS or femoral neck
T-score of at least -2, compared teriparatide, alendronate, and their combination over a 2.5-year period (teriparatide was started at month 6). The teriparatide group had significant increases in LS BMD and femoral neck BMD, which were greater than those in the alendronate and combination groups [
67]. In a study that assessed BMD and fractures for 30 months after a year of exposure to teriparatide, LS and total hip BMD remained significantly higher in the PTH group than in the placebo group, even though the BMDs decreased after discontinuation. When the subjects were divided according to bisphosphonate use, those who took bisphosphonates had an increase in spine and hip BMD, although significant intergroup differences were lost. Among those who did not take bisphosphonates, the BMD decreased. A significant decrease in moderate to severe spine fractures was seen at 18 months of follow-up [
68].
The frequency of transient hypercalcemia within 4 to 6 hours after administration is 10-fold higher among patients who received teriparatide compared with placebo, and in one-third of these, the transient hypercalcemia was reverified on consecutive measurements. The occurrence of leg cramps was also significantly higher in the
teriparatide group compared to the placebo group [
60]. The drug carries a black box warning for osteosarcoma in rats. Teriparatide caused a dose- and duration-dependent increase in this condition among rats treated with the drug. For this reason, children, patients with prior radiation therapy, and those with high bone turnover, such as bone metastasis or Paget disease of bone, should not receive the drug.
In a large clinical trial involving 1,637 postmenopausal women, antibodies to PTH (1-34) developed in 1 woman in the placebo group (<1%), 15 women in the 20 μg/d group (3%), and 44 women in the 40 μg/d group (8%). The antibodies did not have an effect on any of the parameters measured [
60].
Studies examining continuous versus cyclic PTH treatment for osteoporosis have been promising. In one study, 126 women with osteoporosis who were treated with alendronate for at least 1 year were assigned to receive daily subcutaneous PTH (25 μg) or cycles of 3 months of daily PTH followed by 3 months without PTH, or alendronate 70 mg alone for 15 months. There was no significant change in biochemical markers of bone turnover in the alendronate group. In the groups who received PTH, the markers of bone formation increased during the periods when PTH was administered. Bone-resorption markers increased in both PTH groups but more so in the daily-treatment group than in the cyclic-therapy group. The BMD of LS significantly increased by 6.1% in the group who received the daily PTH and 5.4% in the cyclic-therapy group compared to the alendronate group. The difference in LS BMD gains was not significant between the PTH groups. The hip BMD increased marginally in all groups with no significant difference between the groups. The study was not statistically powered to detect a difference in fracture outcomes. The study showed that cyclical administration of PTH resulted in the dissociation of the early anabolic phase of PTH from the subsequent bone remodeling phase allowing for a greater anabolic effect and therefore achieving similar changes in BMD with administration of 60% of the PTH in the cyclic administration group compared to the daily administration group [
69]. Women from this study who were treated with teriparatide and remained at high risk for fracture (17 from the original daily teriparatide group and 15 from the cyclical teriparatide group) were enrolled in a follow-up study after 1 year of alendronate alone to receive a second course of teriparatide (daily) for 15 months while remaining on alendronate. The mean spine BMD increased by 4.7% in the prior daily teriparatide group and by 4.9% in the prior cyclical teriparatide group after retreatment [
70].
While teriparatide is an effective treatment for osteoporosis, its mode of administration can be a limiting factor for some patients. A randomized, placebo-controlled phase 2 study examined the safety and efficacy of transdermal teriparatide patch (doses 20, 30, or 40 μg, worn for 30 min/d) compared to placebo patch and subcutaneous administration of 20 μg/d of teriparatide in 165 postmenopausal women with osteoporosis. Over a 6-month period, the LS BMD increased in all teriparatide patch groups in a dose-dependent manner compared to placebo. At 6 months, mean percentage (SD) change from baseline LS BMD was 2.96%, 3.47%, and 4.97% in the 20-, 30-, and 40-μg transdermal teriparatide groups, respectively. There was a 3.55% increase in LS BMD in the subcutaneous teriparatide group. The BMD change in LS in the group who received the 40 μg/d teriparatide patch was comparable to the BMD increase in the group who received daily subcutaneous teriparatide injections. All groups showed a significant improvement in LS BMD compared with the placebo group. There was a 1.33% increase in total hip BMD in the 40 μg/d teriparatide patch group compared to a 0.09% change in total hip BMD in the subcutaneous teriparatide group at 6 months. The reason for this finding is unclear. The patch showed a higher peak concentration and a shorter half-life compared to
subcutaneously injected teriparatide. Whether the pharmacokinetics of subcutaneous teriparatide resulted in the difference between the two groups is not clear, and further studies are necessary to explore this finding. The change in femoral neck BMD was not statistically significant after 6 months in the treatment groups. BTMs (procollagen type 1 N-terminal propeptide and C-terminal cross-linked telopeptide of type 1 collagen) significantly increased from baseline values in a dose-dependent manner in all teriparatide patch treatment groups compared to placebo patch. The transdermal patch was well tolerated, and there was no significant difference between the adverse events in the transdermal and subcutaneous teriparatide groups. During the 6 months of therapy, no clinically significant hypercalcemia was observed. Additional studies are necessary to further evaluate the efficacy and safety of transdermal teriparatide over a more extended period of time [
71].
Safety Concerns
Teriparatide was approved by the FDA in December of 2002. It carries a “black box” warning for potential increased risk of osteosarcoma, which was observed in high percentage of rodents treated with high doses of teriparatide for most of their lifespan.
The first case of suspected osteosarcoma was reported in 2005 in a postmenopausal female in her 70s with osteoporosis that was diagnosed with metastatic cancer during her 2nd year of treatment with teriparatide. The patient subsequently died, no autopsy was performed, and the primary site of tumor was not identified, but based on bone pathology data, she was diagnosed with osteosarcoma.
A second case was reported in 2010 of a 67-year-old male with history of recurrent prostate cancer. The patient was treated with proton therapy 7 years prior to diagnosis of osteosarcoma of the left pubic ramus. The diagnosis was made after 2 months of treatment with teriparatide. The authors felt that the radiation exposure was the main contributor given the latency period between the time of radiation exposure and diagnosis of osteosarcoma, the occurrence of the tumor within the radiation field, as well as the short time period between teriparatide exposure and diagnosis, which is in conflict with the timeline of tumorigenesis observed in animal studies. However, it is not clear if teriparatide enhanced the development of osteosarcoma in this case. A postmarketing surveillance program for evaluation of an association between osteosarcoma and treatment with teriparatide is ongoing and is expected to continue through 2013 [
72].
Denosumab
Receptor activator of nuclear factor κB ligand (RANKL) binds to its receptor RANK on osteoclasts and osteoclast precursors acting as a key mediator of osteoclast differentiation, action, and survival. This process is regulated by a decoy receptor called OPG that binds RANKL and prevents activation of osteoclasts. Denosumab is a human monoclonal antibody to RANKL that reversibly inhibits osteoclast-mediated bone resorption. Denosumab is FDA approved for the treatment of osteoporosis in postmenopausal women and is administered as a 60-mg subcutaneous injection every 6 months.
In a 2-year randomized, double-blind, placebo-controlled phase 3 study, 332 postmenopausal women with LS
T-scores between -1.0 and -2.5 were assigned to receive either denosumab (60 mg subcutaneously every 6 months) or placebo. The primary end point was change in LS BMD at 24 months. In the denosumab group, the LS BMD increased by 6.5% compared to a 0.6% decline in the placebo group. The total hip, femoral neck, and distal one-third radius BMD were
significantly higher in the denosumab group compared to placebo at 24 months. There was a significant reduction in bone-resorption markers at 1 month and throughout the study in the denosumab group compared to placebo. There was a gradual decline in P1NP, a marker of bone formation, in the denosumab group, which was sustained through the end of the study. There was a transient decrease in serum calcium level compared to baseline after the first dose of denosumab, which subsequently normalized and remained stable thereafter. The overall incidence of adverse events was similar between the two groups; however, there was a higher percentage of patients who reported infection (sore throat) and rash in the denosumab group [
73].
In the randomized, placebo-controlled Fracture Reduction Evaluation of Denosumab in Osteoporosis Every 6 Months (FREEDOM trial), 7,868 women with
T-scores between -2.5 and -4.0 at the LS or total hip were assigned to receive either 60 mg of denosumab or placebo subcutaneously every 6 months for 36 months. The primary end point of the study was new vertebral fracture. There was a 68% relative RR of developing new vertebral fracture (2.3% in the denosumab group and 7.2% in the placebo group), a 40% relative RR of developing hip fracture, and a 20% relative RR in developing nonvertebral fracture compared to placebo. After 36 months, LS BMD increased by 9.2% and total hip BMD increased by 6.0% in the denosumab group compared to placebo. Denosumab decreased serum CTX by 86% at 1 month and by 72% at 6 and 32 months. P1NP levels were also lower compared to the placebo group. There was no significant difference in adverse effects between the groups. There were no cases of ONJ [
74].
The Study of Transitioning from Alendronate to Denosumab (STAND) trial was a phase 3 multicenter, randomized, double-blind study; 504 postmenopausal women with a BMD
T-score between -2.0 and -4.0 on alendronate therapy for at least 6 months were assigned to either continue alendronate therapy or receive denosumab 60 mg subcutaneous every 6 months for a period of 12 months. The primary end point was the percent change in total hip BMD. In the denosumab group, total hip BMD increased by 1.90% compared to a 1.05% increase in the alendronate group. The LS, femoral neck, and distal one-third radius BMD were also significantly higher in the denosumab group compared to the alendronate group. Serum CTX levels were significantly lower in the denosumab group compared to the alendronate group. The safety profile was similar in both groups [
75].
A phase 3, double-blind, multicenter trial compared the efficacy and safety of denosumab (60 mg subcutaneously every 6 months) with alendronate (70 mg weekly) in postmenopausal women with
T-score less than or equal to -2.0 at total hip or LS. Denosumab significantly increased total hip BMD by 3.5% compared to 2.6% in the alendronate (
p < 0.0001) group after 12 months of treatment. A similar pattern was seen at the femoral neck, LS, and distal one-third radius BMD. Serum CTX was significantly lower in the denosumab group until 12 months at which point the decrease in CTX was similar in both groups. P1NP level was significantly lower in the denosumab group throughout the study and at 12 months. The study was not powered to compare fracture rates between the two groups. The overall safety profile was similar for both groups [
76].
Denosumab has also been studied in women with breast cancer treated with adjuvant aromatase inhibitor (AI) therapy. Women with LS, total hip, or femoral neck
T-score between -1.0 and -2.5 on AI (≤6 months or >6 months of treatment) were randomized to receive denosumab or placebo. At 12 and 24 months, the LS BMD significantly increased by 5.5% and 7.6%, respectively, in the denosumab group compared to placebo. The increase in BMD was not influenced by
the duration of AI treatment. The study was not powered to assess treatment effect on fracture rate [
77].
While denosumab is not FDA approved for use in men, a study of men on androgen deprivation therapy for prostate cancer treated with denosumab or placebo for 24 months showed a significant increase in LS, femoral neck, and distal onethird radius BMD compared to placebo. There was also a significant decrease in the incidence of new vertebral fractures (1.5% in the denosumab group vs. 3.9% in the placebo group) [
78].
In the absence of head-to-head trials, it is not possible to compare fracture prevention efficacy of denosumab to other treatments for osteoporosis. Some of the advantages of denosumab include improved patient compliance compared to weekly or monthly oral bisphosphonates, lack of long-term skeletal accumulation, and the ability to use denosumab in patients with renal impairment.
Calcium and Vitamin D Supplementation
In a meta-analysis of 15 trials comparing calcium with placebo, the pooled increase in percentage change from baseline was 2.05% for the total body BMD, 1.66% for the LS, and 1.64% for the hip in patients who received calcium. Vertebral fracture risk decreased by 23% and nonvertebral fracture risk by 14% in the calcium group [
79].
The recommended intake of elemental calcium is 1,000 to 1,200 mg/d for adults older than 50 years. Intake more than 2,000 to 2,500 mg is not recommended, as it may cause hypercalciuria (see section on calcium in “Hypocalcemia”). Vitamin D supplementation has been found to reduce vertebral fractures by 37% in a meta-analysis of 25 trials. A trend was noted toward reduction in nonvertebral fractures as well (RR, 0.72;
p = 0.09). Patients who received hydroxylated forms of vitamin D had larger increases in BMD than did those who received vitamin D
2 [
80]. According to the Institute of Medicine (IOM), the Recommended Dietary Allowance (RDA) for vitamin D is 600 IU daily between the ages of 9 and 70 and 800 IU for people over age 70. The NOF recommends 800 to 1,000 IU of vitamin D a day. The AACE recommends between 1,000 to 2,000 IU of vitamin D a day. Patients with history of malabsorption or bariatric surgery will need higher doses of vitamin D to achieve the desired level. Most experts agree that a minimal level of 30 ng/dl of vitamin D up to 50 to 60 ng/dl is an acceptable target range (also refer to Section on Vitamin D Deficiency).
A recent meta-analysis examined the effect of calcium supplements on the risk of CV events. Eligible studies were randomized, placebo-controlled trials of calcium supplements (≥500 mg/d) and study duration of more than 1 year. The researchers found 143 people allocated to calcium had a myocardial infarction compared with 111 allocated to placebo (hazard ratio 1.31, 95% CI 1.02-1.67,
p = 0.035). While there was a higher incidence of stroke, when the composite end point of myocardial infarction, stroke, or sudden death was examined, the difference did not reach statistical significance. The meta-analysis of trial level data showed similar results. It is important to note that CV end points were not the primary outcomes for these studies, and the studies did not include patients on calcium and vitamin D. Further data are necessary to further evaluate the above findings [
81].
In response to this article, ASBMR issued a statement that numerous large studies of calcium with vitamin D have not shown an increased risk of CV events. It was recommended that patients discuss calcium intake with their health care professional as calcium and vitamin D are important for bone health. It was noted that elderly individuals and those with renal impairment who are on calcium supplementation may be at higher risk of CV complications. The U.S. FDA has begun a safety analysis on calcium supplements.