Merkel cell carcinoma (MCC) is a rare but aggressive carcinoma of the skin, arising most commonly in sun-exposed sites of elderly patients. The diagnosis is based on characteristic histopathologic features. In 2008, the discovery of the Merkel cell polyomavirus led to intensified research into the viral pathogenesisis of MCC. MCC staging guidelines were established in 2010, and it demonstrated the importance of distinguishing clinical vs. pathologic evaluation of lymph nodes in MCC. Surgery and/or radiation is of the mainstay of therapy for early disease, while chemotherapy is reserved for more advanced disease. Treatments based on immunologic mechanisms are currently in development.
Key Points
- •
Merkel cell carcinoma (MCC) is a cutaneous malignancy with an aggressive course.
- •
MCCs has no specific distinctive features, although many present as an asymptomatic red or pink papule or nodule.
- •
The recent discovery of the Merkel cell polyomavirus (MCPyV) offers the potential for understanding the basis of the disease and creating targeted therapies.
- •
The first consensus staging system for MCC was developed in 2010 and emphasizes the importance of sentinel lymph node in staging of disease.
- •
Currently, there are no standardized guidelines for treatment, although surgery with possible adjuvant radiation is favored for local or locoregional disease. Chemotherapy is reserved for distant metastatic disease.
Introduction
MCC is a rare neuroendocrine carcinoma of the skin. MCC was originally described by Toker in 1972 in his report of 5 cases of trabecular cell carcinoma of the skin. Subsequently, through electron microscopy, these tumors were found to contain dense core granules. Because Merkel cells were known to be the only cells in the skin containing these granules, it was hypothesized that trabecular carcinoma of the skin originated from Merkel cells. The exact origin of Merkel cells is still a matter of debate, with some investigators hypothesizing that Merkel cells arise from stem cells of neural crest origin. Other investigators suggest that Merkel cells differentiate from epidermal keratinocyte-like cells (reviewed by Boulais and Misery ). Currently, the former is the favored viewpoint. Other names for MCC have previously been used, including neuroendocrine or primary small cell carcinoma of the skin and anaplastic cancer of the skin.
Although MCC is a rare malignancy, it has an aggressive clinical course. At the time of presentation, 66% of patients have local disease, 27% have nodal involvement, and 7% have distant metastases. The 5-year survival of MCC patients (relative to age-matched and gender-matched controls) is 64% for local, 39% for regional nodal, and 18% for distant metastatic disease. Given the rarity of this malignancy, there is a paucity of prospective trials, and management of MCC remains challenging in the absence of standardized guidelines for treatment. In 2010, the first consensus staging system for MCC was adopted by the American Joint Committee on Cancer (AJCC) ( Table 1 ). Unlike other previously proposed guidelines, a distinction was made between clinical versus pathologic examination of regional lymph nodes. The mainstay of treatment remains surgical, and there is support for the use of adjuvant radiation for localized and regional disease whereas chemotherapy is often reserved for palliative therapy in cases of distant metastases.
| T | N | M |
|---|---|---|
| Tx, primary tumor cannot be assessed | Nx, regional nodes cannot be assessed | Mx, distant metastasis cannot be assessed |
| T0, no primary tumor | N0, no regional node metastasis a | M0, no distant metastasis |
| Tis, in situ primary tumor | cN0, nodes not clinically detectable a | M1, distant metastasis b |
| T1, primary tumor ≤2 cm | cN1, nodes clinically detectable a | M1a, distant skin, distant subcutaneous tissues, or distant lymph nodes |
| T2, primary tumor >2 but ≤5 cm | N1a, micrometastasis c | M1b, lung |
| T3, primary tumor >5 cm | N1b, macrometastasis d | M1c, metastasis to all other visceral sites |
| T4, primary tumor invades | N2, in-transit metastasis e |
| Stage | |||
|---|---|---|---|
| 0 | Tis | N0 | M0 |
| IA | T1 | pN0 | M0 |
| IB | T1 | cN0 | M0 |
| IIA | T2/T3 | pN0 | M0 |
| IIB | T2/T3 | cN0 | M0 |
| IIC | T4 | N0 | M0 |
| IIIA | Any T | N1a | M0 |
| IIIB | Any T | N1b/N2 | M0 |
| IV | Any T | Any N | M1 |
a N0 denotes negative nodes by clinical, pathologic, or both types of examination. Clinical detection of nodal disease may be via inspection, palpation, and/or imaging; cN0 is used only for patients who did not undergo pathologic node staging.
b Because there are no data to suggest significant effect of M categories on survival in MCC, M1a–c are included in same stage grouping.
c Micrometastasis are diagnosed after sentinel or elective lymphadenectomy.
d Macrometastasis are defined as clinically detectable nodal metastases confirmed pathologically by biopsy or therapeutic lymphadenectomy.
e In-transit metastasis is tumor distinct from primary lesion and located either (1) between primary lesion and draining regional lymph nodes or (2) distal to primary lesion.
Introduction
MCC is a rare neuroendocrine carcinoma of the skin. MCC was originally described by Toker in 1972 in his report of 5 cases of trabecular cell carcinoma of the skin. Subsequently, through electron microscopy, these tumors were found to contain dense core granules. Because Merkel cells were known to be the only cells in the skin containing these granules, it was hypothesized that trabecular carcinoma of the skin originated from Merkel cells. The exact origin of Merkel cells is still a matter of debate, with some investigators hypothesizing that Merkel cells arise from stem cells of neural crest origin. Other investigators suggest that Merkel cells differentiate from epidermal keratinocyte-like cells (reviewed by Boulais and Misery ). Currently, the former is the favored viewpoint. Other names for MCC have previously been used, including neuroendocrine or primary small cell carcinoma of the skin and anaplastic cancer of the skin.
Although MCC is a rare malignancy, it has an aggressive clinical course. At the time of presentation, 66% of patients have local disease, 27% have nodal involvement, and 7% have distant metastases. The 5-year survival of MCC patients (relative to age-matched and gender-matched controls) is 64% for local, 39% for regional nodal, and 18% for distant metastatic disease. Given the rarity of this malignancy, there is a paucity of prospective trials, and management of MCC remains challenging in the absence of standardized guidelines for treatment. In 2010, the first consensus staging system for MCC was adopted by the American Joint Committee on Cancer (AJCC) ( Table 1 ). Unlike other previously proposed guidelines, a distinction was made between clinical versus pathologic examination of regional lymph nodes. The mainstay of treatment remains surgical, and there is support for the use of adjuvant radiation for localized and regional disease whereas chemotherapy is often reserved for palliative therapy in cases of distant metastases.
| T | N | M |
|---|---|---|
| Tx, primary tumor cannot be assessed | Nx, regional nodes cannot be assessed | Mx, distant metastasis cannot be assessed |
| T0, no primary tumor | N0, no regional node metastasis a | M0, no distant metastasis |
| Tis, in situ primary tumor | cN0, nodes not clinically detectable a | M1, distant metastasis b |
| T1, primary tumor ≤2 cm | cN1, nodes clinically detectable a | M1a, distant skin, distant subcutaneous tissues, or distant lymph nodes |
| T2, primary tumor >2 but ≤5 cm | N1a, micrometastasis c | M1b, lung |
| T3, primary tumor >5 cm | N1b, macrometastasis d | M1c, metastasis to all other visceral sites |
| T4, primary tumor invades | N2, in-transit metastasis e |
| Stage | |||
|---|---|---|---|
| 0 | Tis | N0 | M0 |
| IA | T1 | pN0 | M0 |
| IB | T1 | cN0 | M0 |
| IIA | T2/T3 | pN0 | M0 |
| IIB | T2/T3 | cN0 | M0 |
| IIC | T4 | N0 | M0 |
| IIIA | Any T | N1a | M0 |
| IIIB | Any T | N1b/N2 | M0 |
| IV | Any T | Any N | M1 |
a N0 denotes negative nodes by clinical, pathologic, or both types of examination. Clinical detection of nodal disease may be via inspection, palpation, and/or imaging; cN0 is used only for patients who did not undergo pathologic node staging.
b Because there are no data to suggest significant effect of M categories on survival in MCC, M1a–c are included in same stage grouping.
c Micrometastasis are diagnosed after sentinel or elective lymphadenectomy.
d Macrometastasis are defined as clinically detectable nodal metastases confirmed pathologically by biopsy or therapeutic lymphadenectomy.
e In-transit metastasis is tumor distinct from primary lesion and located either (1) between primary lesion and draining regional lymph nodes or (2) distal to primary lesion.
Epidemiology
The epidemiology and survival of MCC patients have been studied by several groups using data from the Surveillance, Epidemiology and End Result (SEER) Program of the National Cancer Institute, which included 10% to 14% of the US population observed starting in 1973 and continuing onward as a prospective cohort. The incidence of MCC among the SEER population was reported sporadically before the mid-1980s. Between the years 1986 and 2006, however, the incidence of primary MCC grew significantly, from 0.15 cases per 100,000 to 0.6 cases per 100,000. Similar to the findings from the SEER database, Reichgelt and Visser reported that the incidence of MCC in the Netherlands increased between 1993 and 2007, albeit at a slower rate, from 0.17 to 0.35 cases per 100,000. The increased incidence during this time is at least in part due to improvements in the methods used to accurately diagnose MCC as the immunologic profile specific for MCC has been better elucidated.
Not all groups have found such a dramatic rise in MCC incidence during this same time period; some researchers found a stable incidence since the mid-1990s. In Denmark, Kaae and colleagues used the Danish national health and population registers to assess MCC incidence, and they found that the incidence remained stable between 1995 and 2006 at 0.22 cases per 100,000 person-years. Likewise, the incidence of MCC in Western Australia between 1993 and 2007 increased only slightly for men and did not increase at all for women.
Although the incidence for this population in Western Australia has remained stable, it remains the group with the highest incidence reported worldwide. Girschik and colleagues analyzed histologically confirmed cases of MCC from the Western Australia Cancer Registry from 1993 to 2007 and found an age-standardized incidence over this time frame of 0.82 cases per 100,000. This high incidence corresponds to a high level of UV exposure in this location. The geographic association for MCC development has been studied for the United States as well. The highest age-adjusted incidence in white patients from the SEER database was in Hawaii, which is the geographic location from the SEER database with the highest UV index. Further supporting the causative role of UV in development of MCC is the finding that patients with psoriasis who underwent oral methoxsalen (psoralen) and UVA photochemotherapy have an MCC incidence 100-times greater than the general population.
The majority of patients (71.6%) with MCC are first diagnosed at age 70 or older, and it is infrequently found in patients under age 50. The incidence of primary MCC is higher in men than in women when all ethnic groups are examined, with a ratio of 2:1 in whites and 1.5:1 in other ethnic groups. Men tend to have an initial diagnosis at a slightly younger age than women, with a mean age of 73.6 for men and 76.2 for women at diagnosis. Nearly 95% of the cases reported in the SEER population between 1973 and 2006 occurred in white patients, and only 1.0% and 4.1% were in black and other races (Asian Pacific Islander, American Indian, other), respectively.
Etiology
The groundbreaking discovery by Feng and colleagues in 2008 of a novel polyomavirus associated with the development of MCC has led to several investigative efforts in viral tumorigenesis. In their study, the MCPyV genome was detected in 8 of 10 MCC tumors but only 16% (4/25) of normal skin and non-MCC tumor tissue. The presence of MCPyV in 80% of MCC cases has been substantiated in several studies (reviewed by Kuwamoto ).
Members of the Polyomaviridae family are small, nonencapsulated, circular, double-stranded DNA viruses with the potential to cause transformation in experimental animal models and cultured cells. To date, 17 members of the family have been identified, and 9 are known to infect humans (BK, JC, KI, WU, MCPyV, Human polyomavirus 6, Human polyomavirus 7, Trichodysplasia spinulosa-associated polyomavirus, and Human polyomavirus 9). The first 2 members, BK virus and JC virus, were discovered in 1971. BK virus causes nephropathy in immunosuppressed kidney transplant patients, and JC virus is responsible for progressive multifocal leukoencephelopathy in patients with profound immunosuppression. KI, WU, and TS are not as yet known to cause disease in humans. Simian virus 40 is the most well-known polyomavirus, although its role in human disease and tumorigenesis remains controversial.
The genome of MCPyV is comprised of early and late regions. The former consists of large T-antigen (LT) and small T-antigen regions, which encode proteins necessary for viral replication. The late region encodes viral proteins (VPs) responsible for capsid assembly. LT antigen contains 3 different domains: a binding site for retinoblastoma tumor suppressor protein, a binding site for heat shock protein, and a helicase domain. In Feng and colleagues’ initial study, MCPyV was observed as integrated into MCC tumor genome in a monoclonal pattern, implying viral infection and genome integration occurred before clonal expansion of tumor cells. A follow-up study by Shuda and colleagues identified mutations within the LT antigen in the MCPyV present in MCC tumors. These mutations did not affect the retinoblastoma and heat shock protein binding domains but did result in loss of the helicase domain. As a result, the viral replication capacity of DNA was lost, and the investigators suggested that MCPyV-positive MCC tumor cells underwent selection for LT mutations to prevent autoactivation of integrated virus replication that would be detrimental to cell survival.
MCPyV is ubiquitous in its infection of humans. The seroprevalence of MCPyV antibodies against viral capsid proteins 1 and 2 (VP1 and VP2) ranges from 42% to 88% in healthy adult subjects. In children, the prevalence is 9% under the age of 4, but this increases to 35% by age 13. Although the seroprevalence of antibodies to VP1 and VP2 has been shown higher among MCC subjects, the titers have not been shown to correlate with tumor characteristics or viral load. The presence of a high titer did correspond with a better progression-free survival, although the mechanism of immunoprotection is unclear because MCC tumors do not express VP1 or VP2 proteins. In contrast, another study demonstrated a greater specificity of antibodies directed at MCPyV large and small tumor-associated antigens (T-Ag) among MCC patients. The importance of T-Ag was demonstrated by Houben et al in that its expression is necessary for the maintenance of MCPyV-positive MCC. The seroprevalence of these antibodies was 0.9% among healthy control subjects, while it was 40.5% among MCC patients. Moreover, antibody levels were found to be predictive of clinical course, and therefore Paulson and colleagues concluded that antibodies recognizing T-Ag are specifically associated with MCC, do not effectively protect against disease progression, and may serve as a clinically useful indicator of disease status.
Studies have also been conducted on the cellular immune response to MCPyV. Intratumoral infiltration of CD8+ lymphocytes has been demonstrated as an independent predictor of improved survival among MCC patients. Of 156 cases, patients with robust CD8+ intratumoral infiltration had 100% MCC-specific survival compared with 60% survival among patients with sparse or no CD8+ intratumoral infiltration. This improved prognosis with tumor-infiltrating lymphocytes was recently confirmed by Sihto and colleagues.
The role immunology plays in MCC development is highlighted by the fact that patients with immunosuppression at higher risk for development of MCC. Moreover, immunosuppressed patients with MCC are younger and have more advanced disease at the time of diagnosis. MCC is associated with a diverse number of autoimmune diseases as well as with organ transplantation. A cohort of 309,365 individuals with AIDS was assessed for the development of MCC between −60 to +27 months relative to AIDS onset. Six individuals from this cohort developed MCC, which corresponds to a relative risk of 13.4 (95% CI, 4.9–29.1) compared with the general population, suggesting that a weakened immune system increases the risk of MCC. In Heath and colleagues analysis, 7.8% of MCC patients were found to have some form of immunosuppression, including HIV, chronic lymphocytic leukemia (CLL), and solid organ transplants. A 2009 study examining the Finnish Cancer Registry demonstrated a standardized incidence ratio of 17.9 for developing CLL after a diagnosis of MCC and a standardized incidence ratio of 15.7 for developing MCC among patients already diagnosed with CLL. Another recent study showed that among MCC patients, the standardized incidence ratio was 3.67 for men and 3.62 for women in developing a hematological malignancy. In this study, CLL was the most common at 45%.
Among transplant patients, renal transplant patients most commonly acquire MCC, with the average time span of 7 years between organ transplantation and development of MCC. Thirty-six cases of MCC have been reported to the Cincinnati Transplant Tumor Registry, which translates to an incidence of approximately 3 cases per 1000 transplant patients. The MCPyV may explain the increased incidence of MCC in immunosuppressed patients, but its pathogenesis has not been fully elucidated. The importance of immune status is further highlighted by cases in which MCC tumors have temporarily regressed in transplant patients upon the cessation of immunosuppressive medications.
Presentation
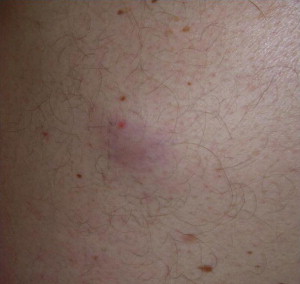
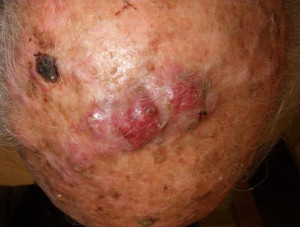
MCC can be a difficult tumor to clinically diagnose, because it tends to be asymptomatic and does not have pathognomonic clinical features. Ultimately, the diagnosis is made by biopsy and histopathologic examination. Typically, lesions present as firm, red-to-purple, nontender papules or nodules ( Figs. 1 and 2 ). In Heath and colleagues study, 56% of patients presented with a tumor having a red or pink hue, whereas 26% had blue/violaceous lesions ( Fig. 3 ). Moreover, 63% of patients in the study reported rapid growth in the size of their tumor over a period of 3 months. Only 11% of patients did not notice any changes in size of primary lesions.
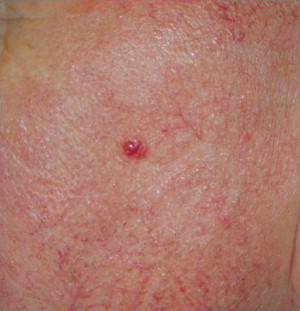
As discussed previously, UV exposure is believed to play a role in the development of MCC. Accordingly, MCC is most commonly located on UV-exposed areas, such as the head and neck, which account for 48% of all MCC diagnoses. Incidence of MCC of the upper limb is 19%, followed by a 16% incidence of MCC of the lower limb and 11% incidence of MCC of the trunk. UV-protected locations, such as the trunk, back, and buttocks, are less commonly affected than UV-exposed sites. The MCC site with the best prognosis is presentation on the limbs, which generally correlates with less advanced disease. MCC presentation on the trunk is associated with distant metastasis at the time of diagnosis.
Some unusual primary sites for MCC to occur include mucosal sites, including the lacrimal gland and the parotid gland. Approximately 5% of all cases of first primary MCC present in mucosal sites. From 1992 to 2001, the total number of cases of first primary MCC was 1027 and of these cases, 50 had an initial presentation in a mucosal anatomic site. The most common sites affected by mucosal MCC are the larynx, followed by the nasal cavity, pharynx, and mouth. There have also been rare reports of vulvar and penile MCC. Mucosal MCC is associated with a worse prognosis than primary cutaneous MCC at other anatomic sites. Part of the reason for the worse prognosis may lie in the difficulty of detection and delayed diagnosis.
Subcutaneous primary tumor is a less common presentation of MCC (see Fig. 4 ). Reports in the literature describe a subcutaneous nodule or mass with minimal to no overlying epidermal change as the initial presentation. The sites of these nodules have included the cheek and the arm. Differential diagnoses for these nodules and masses have included lipoma, carcinoid, epidermal cyst, and cutaneous metastases until pathologic examination rendered the diagnosis of MCC.
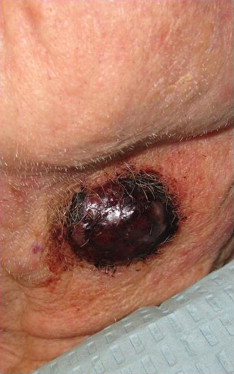
Another less common presentation of MCC is an enlarged lymph node without any findings of primary cutaneous disease. In Heath and colleagues’ series, nodal MCC with an unknown primary was reported as the initial presentation in 14% of MCCs. Of reported cases, the most common site of MCC presenting in the lymph node was inguinal, although axillary, submandibular, and retroperitoneal sites also were reported. The prognosis of these primary nodal presentations of MCC is speculated as being similar to those of primary cutaneous MCCs with nodal metastasis. The origin of these nodal MCCs is not entirely understood. The general prevailing viewpoint is that these nodal MCCs represent nodal metastasis from a regressed primary cutaneous MCC. An alternative theory is that lymph nodal MCCs are primary sites of tumor formation, but given that Merkel cells have never been localized as primary cells of lymph nodes, this theory is less favorable. Because distinction from other poorly differentiated small cell or neuroendocrine tumors may be difficult, stringent pathologic techniques must be used to diagnose MCC presenting in a lymph node without a known corresponding cutaneous lesion.
Diagnostic evaluation
Pathology
Histopathologic features
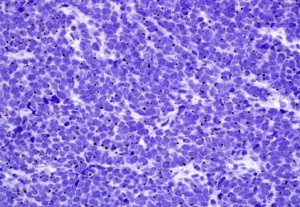
All neuroendocrine tumors (eg, MCC, small cell lung carcinoma, and carcinoid tumors) share similar histopathologic features, featuring small to medium-sized cells with round nuclei and scant cytoplasm. The nuclei have finely granular or stippled chromatin, sometimes exhibiting a smudged appearance with inconspicuous nucleoli ( Fig. 5 ). The high nucleus:cytoplasm ratio imparts a blue or basaloid appearance at scanning magnification. Some MCCs have cells with more vesicular chromatin with multiple small nucleoli, irregular nuclear contours, and more abundant cytoplasm. The latter two features were associated with a lack of detectable MCPyV infection in one small study. Nuclear pleomorphism is typically mild, whereas mitotic figures and apoptotic cells abound (see Fig. 5 ).
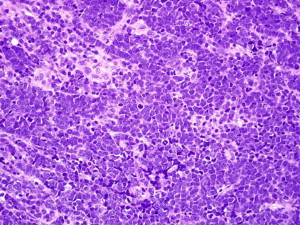
MCC is divided into subtypes based on growth pattern and cell size. Most MCCs have a nodular architecture with multiple, interconnecting aggregations of cells forming a tumor centered in the dermis with infiltrative strands of cells at the tumor periphery. The presence or absence of these infiltrative strands is used to classify MCCs into infiltrative or nodular patterns. The so-called diffuse pattern consists of large collections of neoplastic cells coalescing into sheet-like aggregations of cells. The trabecular pattern, for which MCC was originally named trabecular carcinoma by Toker in 1972, consists of interconnecting strands of cells ( Fig. 6 ). Epidermal involvement can be seen in any of these subtypes and occurs in approximately 10% of all cases. Purely intraepidermal MCC, also called MCC in situ, is rare. Combinations of these patterns frequently occur in the same tumor and, with the exception of improved survival associated with cases lacking an infiltrative pattern, separation based on growth pattern does not seem to have clinical significance.
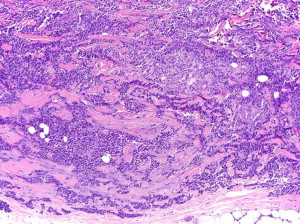
In addition to architectural subclassification, MCC is classified according to cell size. The majority of MCCs are the intermediate cell type, with medium-sized nuclei (10–15 microns), which are significantly larger than nearby lymphocytes (see Fig. 5 ). Some degree of nuclear molding is present, often manifesting as the ball-in-mitt pattern with compression of some nuclei into crescent shapes around adjacent circular nuclei. The small cell variant of MCC features cells approximately the size of lymphocytes, which can lead to misclassification of the tumor as a lymphocytic process. An uncommon large cell variant with greater nuclear pleomorphism is also described.
Immunohistochemical and ultrastructural features
Due to histopathologic overlap with neuroendocrine tumors, hematologic malignancies, poorly differentiated carcinomas, and various other neoplasms, confirmatory studies are used in the vast majority of suspected MCCs. Electron microscopy played a critical role in identifying MCC as a neuroendocrine carcinoma and can be used as an ancillary test to confirm the diagnosis. The presence of paranuclear and/or cytoplasmic bundles of tonofilaments (keratin intermediate filaments), cytoplasmic dense-core granules, and desmosomal attachments is characteristic of MCC. Currently, widespread access to immunostains, which offer rapid results with good sensitivity and specificity, has made immunostaining the confirmatory test of choice for MCC.
Although many immunostains have been studied in MCC, cytokeratin 20, low-molecular-weight cytokeratins (eg, CAM5.2), and neurofilaments have a high sensitivity for MCC and are the most frequently used immunostains in this differential diagnosis. A paranuclear dot staining pattern is characteristic of MCC ( Fig. 7 ). Diffuse cytoplasmic staining is less specific. Negative staining with thyroid transcription factor 1 and cytokeratin 7 can be used to increase specificity, especially when differentiating from small cell lung carcinoma. Neuroendocrine markers, such as synaptophysin, chromogranin, and neuron-specific enolase, are frequently used, but these are nonspecific and of limited diagnostic value. In exceptionally challenging cases, chromosomal analysis with array-based comparative genomic hybridization can be helpful.
Prognostic features
Tumor size and thickness, along with lymphovascular invasion, have prognostic significance in MCC. Intravascular invasion occurs in 56% to 93% of cases and sometimes requires a vascular immunostain (eg, D2-40) for identification. Promising data have shown positive p63 staining correlates with a remarkably worse prognosis, but most prognostic studies of immunostains, including MCPyV large T antigen, Ki-67, p53, and Rb, are small and have inconsistent results.
Staging
Several staging guidelines for MCC had been developed before 2010. Because of the overall paucity of cases of MCC, however, none of these cohorts included more than 251 cases. In 2010, the first consensus staging system was published based on 5823 cases registered in the National Cancer Data Base (a US-based registry founded by the American Cancer Society and the American College of Surgeons) ( Table 1 ). The staging system has subsequently been adopted by the AJCC and the International Union Against Cancer.
The new consensus staging system has 4 stages determined by tumor size (T), the presence or absence of metastases to regional lymph nodes (N), and the presence or absence of distant metastases (M). For local disease, a distinction was made between those primary tumors less than or equal to 2 cm in size (T1) at the time of presentation, which showed a 66% 5-year survival. In contrast, those tumors between 2 cm and 5 cm in size (T2) carried a 51% 5-year survival. The T3 designation was given to those tumors that exceeded 5 cm in size. Although the prognoses of these patients were similar to those of those having T2 disease, this format was chosen so that the T4 designation could be assigned to those tumors invading deeper underlying structures so as to maintain congruence with other AJCC staging systems.
The staging system also takes into consideration whether examination of regional lymph nodes has occurred clinically or pathologically. Data in the cohort demonstrate a worse prognosis for those with undetectable lymph nodes on clinical examination alone compared with those who have pathologically proven negative lymph nodes. Accordingly, those with pathologically negative lymph nodes are categorized as having either stage IA or stage IIA disease. In contrast, those with negative lymph nodes based on clinical examination alone are regarded as having either stage IB or stage IIB disease. Sentinel lymph node biopsy (SLNB), therefore, should be considered for all patients presenting with clinically localized disease.
For distant metastases, staging criteria are similar to that of melanoma. Distinctions are made between having no metastatic disease, metastases to distant skin, subcutaneous tissue, or lymph nodes, metastases to lung, or metastases to any other visceral site.
Prognosis
The prognosis of MCC is generally poor. Stage at time of presentation is the most important prognostic indicator. Under the revised staging guidelines (discussed previously), patients with local disease have a 5-year survival of 66% if the primary tumor is less than or equal to 2 cm in diameter, whereas those who present with tumors greater than 2 cm have a 5-year survival of 51%. Patients who have local disease confirmed with pathologically negative lymph nodes have a better prognosis (76% at 5 years) compared with those who have negative lymph nodes based on clinical examination alone (59% at 5 years). Among those patients with metastases to regional lymph nodes, the 5-year survival ranges from 26% to 42%. Patients with distant metastases have a prognosis of 18% survival at 5 years.
In addition to stage at the time of diagnosis, clinical features have been evaluated as potential prognostic factors. Smith and colleagues reported that in a review of 2104 MCCs in head and neck locations, the lip had a significantly worse prognosis with a higher rate of invasion into deeper underlying structures and a higher rate of nodal metastasis. For MCCs of any location, a primary tumor presenting on the trunk is associated with a worse prognosis. Men have been found to have a worse prognosis compared with women. Older patients (>70 years old) have a worse prognosis compared with their younger counterparts. A recent review of a database of 500 MCC cases demonstrated a strong association between lymphovascular invasion by MCC and disease-specific death.
Imaging
Currently, there are no consensus guidelines regarding which patients should be imaged after an initial diagnosis of MCC. Although SLNB is useful for detection of microscopic locoregional metastasis, imaging may be useful as an adjunct to determine the presence of distant metastasis. In general, those patients with clinically localized disease and no clinical evidence of metastatic nodal disease should be assessed first for candidacy for SLNB with possible adjunctive imaging for evaluation and staging. Those with evidence of nodal or metastatic disease on initial history and physical examination are recommended to have preoperative imaging to evaluate for distant metastasis and facilitate staging at the time of MCC diagnosis.
The preferred modality for imaging MCC has yet to be established. Conventional imaging has primarily been with CT, ultrasound, and MRI. More recently, the role of (18)F–fluorodeoxyglucose positron emission tomography (FDG PET) alone or in combination with CT (FDG PET–CT) has been investigated. Yao and colleagues reported 2 cases in which FDG PET detected metastatic disease in subcentimeter nodes that were not detected on initial CT scans. The investigators argue that FDG PET has increased sensitivity compared with conventional CT in identifying these small foci of metastases. Similarly, Lin and colleagues reported the increased sensitivity of FDG PET to detect recurrent MCC compared with conventional CT. A retrospective review of 18 patients demonstrated that all proven sites of MCC greater than 5 mm were visualized on FDG PET–CT, and these findings led to changes in management of 9 patients. In a study of head and neck cancers, Shintani and colleagues determined that 2 of 5 MCC patients had their postsurgical adjuvant treatment plan change as a result of findings on PET CT. Two recent case series, however, comparing the utility of FDG PET–CT to traditional imaging modalities showed no demonstrable difference in sensitivity or specificity between the two. However, FDG PET–CT has greater sensitivity in detecting bony metastases for more common tumors, such as lung and colorectal carcinoma. This may be applicable to neuroendocrine tumors over traditional imaging for MCC.
The frequency of imaging is also a matter of controversy. Given that the median time to recurrence of MCC is 9 months and that 90% of MCC recurrences occur within the first 2 years of diagnosis of the primary tumor, more frequent surveillance when clinically indicated during this period is advocated. Currently there is no standardized algorithm for the role of imaging in monitoring for recurrence. At the authors’ institution, the preferred imaging modality is FDG PET–CT, which is used to screen high-risk patients or those with clinical evidence of metastasis at the time of primary MCC diagnosis. The frequency of follow-up is handled on an individual basis using FDG PET–CT, although patients are typically reimaged every 6 months for the first 2 years.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree