and Jeffrey E. Gershenwald1
(1)
Department of Surgical Oncology, The University of Texas MD Anderson Cancer Center, Houston, TX, USA
Chapter Overview
Many important issues are relevant to melanoma survivors after initial melanoma diagnosis and treatment. A diagnosis of melanoma not only affects patients during the time of initial treatment, but also may play a significant role in their life for many years. These issues are broad and apply to many aspects of patients’ lives. This chapter will discuss overall and stage-specific surveillance strategies and methods for risk reduction and prevention that are important to the melanoma survivor population. It is also important for patients and care providers alike to understand and learn to manage sequelae that may accompany various melanoma treatments, from surgery to targeted therapies. Finally, this chapter will discuss issues relating to long-term survivorship such as psychosocial adjustment, reproductive issues, and health care costs.
Introduction
The goal of this chapter is to address topics relevant to melanoma survivors. A diagnosis of melanoma not only affects patients during the time of initial treatment, but also may play a significant role in their life for many years. Treatment sequelae may remain and factor into quality of life over time. The need for ongoing surveillance, the potential psychological and social effects of the diagnosis, and the need for associated treatments can have a substantial effect on an individual patient’s life. Therefore, our goal is to establish a streamlined longitudinal plan for melanoma patients that addresses issues related to melanoma surveillance and monitoring, makes recommendations specific to melanoma treatments, and outlines approaches to optimize overall patient health, well-being, and quality of life.
Epidemiology
An estimated 76,100 people will be diagnosed with melanoma in 2014, and 9,710 patients will die of this disease (American Cancer Society 2014). Overall, the incidence of melanoma is increasing worldwide; in the United States specifically, the incidence has been rising by an average of 2.6% per year. Increasing awareness has led to both earlier detection and earlier treatment of melanoma. Given the increasing success of early identification and surgical treatment of melanoma, the overall number of melanoma survivors requiring surveillance has increased over time. It is estimated that almost one million melanoma survivors can be identified in the United States and an estimated 1.3 million melanoma survivors are expected to be living in the United States by 2022 (American Cancer Society 2013). A current challenge for both patients and providers is the fact that after the initial diagnosis and treatment of melanoma, surveillance and subsequent care for melanoma survivors may become fragmented between specialized cancer centers and primary care physicians. Efforts to create longitudinal guidelines for the delivery of evidence-based care to be followed during patient transition to survivorship are clearly needed; such guidelines must also continue to evolve. Overall, these guidelines should be simple and streamlined so that they can be applied to patients in a variety of clinical settings.
Melanoma survivors are generally aware of their stage-specific risk for recurrence. However, it is important to note that survivors also have an increased risk of developing a second or subsequent primary melanoma, compared with the risk of developing primary melanoma in the general population. Patient risk stratification can potentially guide recruitment of patients at high risk into more intensive screening regimens or even prevention trials. Although comprehensive integrated risk models are clearly warranted, it is well known that the risk factors of fair skin, increasing age, male sex, tanning bed use, sun exposure, and family or personal history of melanoma or nevi can also be used to stratify patients. Although more refinement is needed, this stratification can guide initial screening and inform posttreatment surveillance strategies for patients with a new primary melanoma.
Classification
Superficial spreading melanoma is the most common histologic subtype of melanoma (70% of cases); it usually occurs in the setting of a preexisting nevus. Nodular melanoma is the next most common type, accounting for 15–30% of all cases of melanoma. Lentigo maligna melanoma represents 4–10% of all cases of melanoma; it usually occurs in sun-exposed areas and is commonly found on the faces of elderly white women. Lentigo maligna melanoma is generally slow to become invasive, and a delay in initial diagnosis is not unusual. Acral lentiginous melanoma occurs more commonly in dark-skinned patients (35–60% of cases of melanoma occurring in dark-skinned individuals); it usually occurs on the palms, soles, and nail beds. Finally, in a small proportion of cases of melanoma, the lesion is amelanotic, lacks pigmentation, and is generally more difficult to diagnose.
In addition to the aforementioned histologic classifications, several molecular aberrations have been identified that can be used to classify and stratify patients with cutaneous melanoma. Molecular-based classifications of melanoma are now routinely used and are important in identifying tumors that, for example, may respond differently to specific treatment regimens than would other melanoma subtypes, or that may not respond to certain treatments at all. This is of particular relevance to patients with metastatic disease because these molecular criteria may ultimately help to determine the most appropriate treatment regimen for a specific tumor. In approximately 40% of cases of superficial spreading melanoma, a mutation in the BRAF gene is present, in most cases (>85%) a point mutation at V600E, which leads to a 400-fold increase in the activity of the BRAF protein, a serine/threonine protein kinase. However, approximately 80% of benign nevi also harbor BRAF mutations, suggesting that this mutation alone cannot account for the malignant potential of superficial spreading melanoma. Nonetheless, tremendous advances in the treatment of metastatic melanoma have been made by using therapies that target the BRAF mutation, contributing to the overall growing excitement about targeted therapies in melanoma. There is currently great interest in directing the growing knowledge of the molecular biology of melanoma toward new, more focused, and personalized therapeutic approaches.
Definition of Survivorship
A cancer survivor is anyone who has been diagnosed with cancer, from the time of initial diagnosis and treatment through the remaining years of life. The goal of survivorship care is to prevent, detect, and manage complications that arise from cancer or cancer treatments and to improve overall health and quality of life for survivors. The experience of living with a cancer diagnosis varies greatly from individual to individual. Some survivors may live with cancer as a chronic disease requiring periodic treatments, and others may enjoy long-term remission. Many survivors lead normal lives with minimal, if any, residual side effects.
It is important to note that the diagnosis of melanoma and associated initial treatments do not represent the end of the cancer experience. For most melanoma survivors, ongoing surveillance is critically important because of the increased risk for a second primary melanoma in addition to the stage-specific risk for recurrence. Additionally, the delayed sequelae of melanoma treatments may continue to affect the survivor’s quality of life. Recovering from the social and emotional strain of cancer is an ongoing process that requires sensitivity on the part of health care providers to the stresses on both the patients and their families. Physicians and care teams now realize that many challenges remain to be addressed in helping survivors achieve good quality of life, even after the specific treatment(s) for melanoma have ended.
MD Anderson Mission
The survivorship mission at MD Anderson is three-fold: (1) to address the outcomes of cancer and cancer treatment; (2) to improve cancer survivors’ health; and (3) to improve the quality of life of cancer survivors through integrated programs focusing on patient care, research, prevention, and education. Family, friends, and caregivers are also included in the process because they, too, are involved in the care of cancer survivors.
To facilitate the transition from initial cancer patient to survivor, a detailed survivorship care plan is very helpful. The goal of the survivorship plan is to effectively communicate information about the patient’s diagnosis, treatment, and possible adverse effects associated with the treatment, including late adverse effects, to the patient and the patient’s subsequent physicians to manage symptoms, optimize surveillance, and align expectations between the patients and the care team.
The components of the survivorship plan are cancer surveillance, risk-reduction strategies, management of late effects of therapy, and support for the psychosocial aspects of survivor treatment and long-term care. At MD Anderson, our goal is to establish guidelines that contribute to the continuity of care among the oncologic teams, consultants, and primary care physicians, as well as patients and their families, to allow individuals to successfully and smoothly transition to survivorship care.
Cancer Surveillance
Two essential elements of melanoma follow-up care are early detection of a new primary melanoma and detection of recurrent disease. Patients with a history of melanoma have an increased risk of developing a second primary melanoma, as well as a stage-specific risk of disease recurrence. The likelihood of recurrence depends on the stage and substage of the initial melanoma (Baughan et al. 1993); patients who present with early-stage disease have a lower likelihood of recurrence than those who present with advanced disease. A study of patients with melanoma at a large melanoma unit demonstrated recurrence rates of 5% for appropriately treated stage IA, 18% for stage IB, 29% for stage IIA, 40% for stage IIB, and 43% for stage IIC melanoma, after a median follow-up period of 6 years (Francken et al. 2008). In terms of survival, the 5-year disease-free survival rate for patients with stage I melanoma has been shown to be >90% (Kalady et al. 2003), and the 5-year recurrence-free survival rate for patients with stage III melanoma can vary substantially.
In both early-stage and late-stage disease, risk stratification is influenced by multiple factors, including the burden of disease in the regional lymph node basins. In patients with stage III melanoma, a distinction is made between those with micrometastatic disease and those with macrometastatic disease because the median 5-year overall survival rate of patients with micrometastases is approximately 70%, whereas among those with macrometastases, the median 5-year overall survival rate decreases to 43%. However, outcomes vary even within the overall cohort of patients with micrometastatic disease; 5-year overall survival rates range from 23% to 87% (Balch et al. 2010). Multivariate analyses have demonstrated that overall survival rates also vary with the thickness of the primary tumor, mitotic rate, patient age, tumor ulceration, anatomic site, and patient sex. Therefore, each patient must be individually assessed and stratified according to the patient’s particular risk factors. It has also been noted that both primary and recurrent melanoma is often detected by patients rather than by medical practitioners (Moore Dalal et al. 2008; Romano et al. 2010). Therefore, patients should be active participants in their follow-up visits and should be encouraged to be involved in their own ongoing surveillance.
A 2003 study of the Surveillance, Epidemiology, and End Results database demonstrated that the risk of developing a second primary melanoma is high within this patient population; the risk of synchronous primary melanoma at the time of diagnosis was 0.5%, whereas the risk of metachronous primary melanoma was 1% at 1 year, 2% at 5 years, 3% at 10 years, and 5% at 20 years (Goggins and Tsao 2003); overall, these data highlight the importance of comprehensive skin examinations as part of overall surveillance.
Nieweg and Kroon (2006) demonstrated that approximately one-third of patients whose melanoma is treated locally for site-specific or regional lymph node recurrence can be treated until there is no evidence of disease with additional surgery or other therapies. The overall 5-year relapse-free survival rate for patients with stage III melanoma ranges from 63% for stage IIIA disease to 11% for stage IIIC disease (Romano et al. 2010). Although median 5-year overall survival rates for patients with stage IV disease have historically been only about 10%, a growing subset of patients with stage IV melanoma, particularly in the era of recently developed therapies such as immune modulators, achieve durable long-term survival. Overall, patients with early-stage disease have a low risk of recurrence but still have an increased risk of developing a second primary tumor. Patients whose primary disease is more advanced also have an increased risk of developing a second primary tumor, but they also have a higher risk of recurrence than patients with early-stage disease. In either case, close surveillance is warranted to evaluate any site of new disease or evidence of recurrence or metastasis.
In addition to having an increased risk of developing a second primary melanoma or recurrent disease, melanoma survivors may have an increased risk of developing other types of cancer, including breast cancer, prostate cancer, and non-Hodgkin lymphoma (Bradford et al. 2010). Therefore, survivorship programs should incorporate appropriate screening regimens for these patients and make efforts to optimize their overall health maintenance beyond single-organ or disease-specific care.
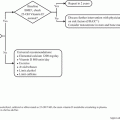
The melanoma surveillance plan proposed depends on the pathologic stage of the melanoma at the initial diagnosis and the interval of disease-free survival. Initial surveillance includes a clinical visit and physical examination of the skin and regional lymph node basins every 3–12 months, depending on the pathologic stage of the melanoma. At MD Anderson, patients have been broadly categorized into four groups for timing of transition into our survivorship program; each group has stage-specific surveillance strategies. Category 1 patients have stage 0 melanoma (i.e., melanoma in situ) and no evidence of disease after 6 months. Routine surveillance, which is initiated at 1 year after completion of treatment, includes an annual dermatologic examination and lymph node basin survey. Category 2 patients, who have stage IA melanoma and a disease-free interval of 3 years, are followed up with annual skin examinations and self-inspection. Category 3 patients have stage IB-II melanoma and no evidence of disease after 5 years, and category 4 patients have Stage III-IV disease and no evidence of disease after 5 years. Category 3 and 4 patients make annual visits to the clinic to undergo a skin examination and nodal basin survey. Category 4 patients may also receive interval radiologic imaging (e.g., chest x-ray, computed tomography scan, positron emission tomography/computed tomography scan) depending on their clinical history.
The use of imaging in surveillance has been studied, but results and recommendations of these studies have varied. For example, National Comprehensive Cancer Network guidelines recommend that a chest x-ray, computed tomography scan, or positron emission tomography/computed tomography scan be considered every 3–12 months to screen for recurrent or metastatic disease in patients with stage IIB-IV disease within the first 5 years of completion of treatment. After that time, if the patient is asymptomatic, routine radiologic imaging is not recommended. Additionally, annual brain magnetic resonance imaging may be considered in this group of high-risk patients (National Comprehensive Cancer Network 2013). The potential benefits of imaging must be balanced with the risk of false-positive results that may increase patient anxiety and necessitate unnecessary and potentially harmful interventions.
Several studies have examined the utility and cost-effectiveness of imaging for detection of pulmonary metastases in patients with melanoma (Pandalai et al. 2011). A cost-effectiveness analysis recommended against frequent use of chest x-rays given the low detection rate compared with the cost burden (Mooney et al. 1997), whereas other groups have recommended evaluation with a chest x-ray every 6–12 months for patients with melanoma (Morton et al. 2009). Additionally, ultrasound has increasingly been used for nodal basin surveillance, with reported sensitivities ranging from 24% to >80% (Machet et al. 2005; Uren et al. 2007; Boland and Gershenwald 2012); additional studies are needed to assess the role of ultrasound in surveillance.
Risk Reduction and Prevention
The risk of developing a subsequent primary melanoma or other new malignancy is increased in patients who have been diagnosed with and have previously been treated for melanoma, compared with the general population. Therefore, risk reduction and prevention strategies should focus on patient education, surveillance, and screening for skin malignancies. In addition, the toxicities of systemic and local melanoma treatments may increase the risk of other medical problems, such as cardiovascular disease or osteoporosis, which should be kept in mind during surveillance and follow-up. Given the possible increased risk for other cancers, routine cancer screening protocols should be considered for melanoma survivors.
Health maintenance strategies, such as routine gynecologic and breast or prostate screening, as well as weight loss counseling, tobacco cessation, and routine vaccines, are also important in preserving the overall health and well-being of survivors. The goal of a survivorship program is optimal health with the best possible quality of life for each individual survivor. At MD Anderson, survivors are enrolled in dermatologic screening programs, referred for gynecologic and breast or prostate screening as appropriate, offered counseling regarding diet or weight management and tobacco cessation programs as applicable, and provided with recommendations for routine vaccinations as needed.
Patient-guided, self-administered skin examinations are recommended and should focus on identification of new skin lesions or those changing in size, shape, or color. The ABCD rule can help distinguish an abnormal mole from a normal mole: A is for asymmetry, B is for border irregularity, C is for irregularity in color, and D is for diameter (particularly if larger than 0.25 in). However, not all cases of melanoma fit these guidelines, and skin lesions that do not heal, spread color from one area to the surrounding area, develop new redness beyond the border of the lesion, become itchy or tender, or develop surface changes should prompt dermatologic evaluation.
Risk-reduction strategies specific to melanoma, including avoiding sun exposure, should be emphasized to melanoma survivors (Table 13.1). A sampling of melanoma survivors suggests that they tend to use sunscreen and avoid exposure to ultraviolet light more than does the average person, but significantly less than expected in a population known to be at high risk. On the basis of these observations, there is clearly room for improvement in educating and counseling melanoma survivors about risk-reduction strategies, including avoiding sun exposure and tanning beds.
Table 13.1
Strategies for reducing your risk of melanoma
Avoid sunburn. |
Limit sun exposure. |
Do not use tanning beds or other artificial sunlight sources. |
Wear a sunscreen rated at least SPF 30, a broad-brimmed hat, and a long-sleeved shirt when you are outside. |
Wear sunglasses when you are outside. |
Stay inside during the sun’s peak hours between 10:00 am and 3:00 pm. |
Protect children. Infants younger than 6 months should be completely shielded from direct sun exposure. Apply sunscreen to infants older than 6 months, and teach older children to make applying sunscreen a regular habit before they go outside. |
Examine your skin monthly. Have any suspicious moles checked by a health care practitioner. |
If you are at risk, have your skin examined at least once each year by a dermatologist. |
Melanoma Treatments and Their Sequelae
Surgery
Initial surgical management of early-stage primary melanoma in patients with clinically negative lymph nodes includes wide excision of the primary tumor with margins appropriate for tumor thickness (Ross and Gershenwald 2011). Evaluation of the regional nodal basins at risk may also be performed using the technique of lymphatic mapping and sentinel lymph node biopsy. The decision to perform sentinel lymph node biopsy is primarily based on the histologic characteristics of the primary tumor (Gershenwald and Ross 2011; Boland and Gershenwald 2012; Gershenwald et al. 2012). For patients with evidence of regional nodal disease, a completion lymph node dissection represents the current recommended standard practice, although trials are currently underway to assess whether this is required in all patients with sentinel lymph nodes positive for disease (Morton 2012).
Patients who undergo sentinel lymph node biopsy may experience some regional numbness or hyperesthesia owing to sensory nerve damage during surgery, although this is usually relatively minor and well tolerated by most patients. Of more concern is the potential for lymphedema, which can occur after sentinel lymph node biopsy but is more common after formal complete lymph node dissection. Interim analyses of the Multicenter Selective Lymphadenectomy Trial-I, an international randomized clinical trial comparing outcomes of patients who undergo wide excision of the primary site and observation of the regional nodes with the outcomes of patients who undergo wide excision of the primary site and sentinel lymph node biopsy, have thus far demonstrated a lower incidence of lymphedema in patients for whom lymphadenectomy was performed for early, clinically node-negative disease (i.e., those who underwent sentinel lymph node biopsy after a sentinel lymph node was found to be positive for disease) compared with those who underwent delayed lymphadenectomy for clinical regional node recurrence after wide excision alone (Faries et al. 2010). Thus, early detection and disease treatment are a primary goal for melanoma care for both oncologic and quality-of-life reasons.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree