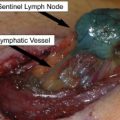
Histologic analysis remains the gold standard for diagnosis of melanoma. The pathology report should document those histologic features important for guiding patient management, including those characteristics on which the diagnosis was based and also prognostic factors. Pathologic examination of sentinel lymph nodes provides very important prognostic information. New techniques, such as comparative genomic hybridization and fluorescence in situ hybridization are currently being studied to determine their usefulness in the diagnosis of melanocytic lesions. Recent molecular studies have opened new avenues for the treatment of patients with metastatic melanoma (ie, targeted therapies) and molecular pathology is likely to play an important role in the emerging area of personalized melanoma therapy.
Pathology is a key aspect in the multidisciplinary care of patients with melanoma. The initial definitive diagnosis of primary melanoma is usually established by pathologic examination of a tissue biopsy. The pathologic assessment of various tumor parameters enables the most accurate estimation of prognosis in early clinical stages and determines the most appropriate next stages of further clinical management. Pathologic assessment of any potential or likely metastasis is also critical. To both reduce the risk of diagnostic error and to facilitate optimal patient management, it is important that clinicians are aware of potential pitfalls and limitations of pathologic assessment. In the past decade, new insights into the molecular pathogenesis of melanoma have been obtained and these are now being harnessed clinically to improve patient management. For example, molecular pathology is now used to enhance melanoma diagnosis, classification, prognostication, and to predict responsiveness to novel promising selective targeted therapies for melanoma.
Pathologic diagnosis of melanoma
Although melanoma may be strongly suspected from the clinical features of the tumor, definitive diagnosis requires pathologic examination of a tissue biopsy. Melanoma can usually be diagnosed accurately, rapidly, and reproducibly by routine pathology. Pathologic diagnosis rests on balancing at least 20 architectural and cytologic features together with those of any host response and must be correlated with clinical features such as the age of the patient and anatomic site of the lesion. A credible diagnosis requires accurate pathologic assessment, awareness of potential pitfalls, and the ability to make a logical and reasoned judgment from all the information available.
Accurate assessment and documentation of important pathologic variables critically influence the management of patients with melanoma. However, of even greater importance is the need to accurately determine whether a cutaneous melanocytic lesion is benign or malignant (ie, a nevus or a melanoma). For this reason, pathology reports of melanocytic lesions should (1) document the key criteria on which the diagnosis was based, and (2) provide the pathologic prognostic and other parameters important for patient management ( Box 1 ).
Diagnostic summary
Excision of skin lesion from right arm: melanoma, AJCC (2010) pT3b, pNX, margins clear
Supporting information
Clinical
Site and laterality: right arm
Clinical diagnosis:? melanoma arising in a dysplastic nevus
Specimen type: excision biopsy
Previous treatment/trauma: nil known
Previous melanoma: nil
Distant metastasis: nil known
Other medical history: none relevant
Comment: suspicious area marked by suture
Macroscopic
Size of specimen: 35 mm × 15 mm
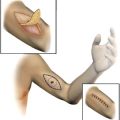
Description: an ellipse of skin, 35 mm × 15 mm, bearing an irregularly pigmented plaque 14 mm × 11 mm. This shows irregular margins with central ulceration. A central 3-mm area of increased pigmentation has been marked by a suture. The lesion is 2 mm from the nearest superficial margin.
Other lesions: N/A
Microscopic
Diagnosis: primary melanoma, invasive
Tumor thickness: 2.3 mm
Excision margins:
Invasive, clear, 3.7 mm
In situ, clear, 1.4 mm
Deep, clear, 5.0 mm
Ulceration (mm diameter): present (2.0 mm)
Mitotic rate (/mm 2 ): 6
Microsatellites: absent
Level of invasion (Clark): IV
Lymphovascular invasion: absent
Tumor-infiltating leucocytes:
Distribution: focal
Density: sparse
Intermediate/late regression: absent
Desmoplasia: absent
Neurotropism: absent
Associated benign nevus: dysplastic compound nevus
Intraepidermal growth: mixed lentiginous and pagetoid
Subtype: superficial spreading
Description: the sections show skin and underlying subcutis with an asymmetric compound melanocytic tumor. The epidermal component shows lentiginous and focally nested growth of large epithelioid melanocytes with variable intracytoplasmic pigment. There is focal single-cell and nested pagetoid epidermal invasion by large atypical cells mainly in the central part of the tumor. The dermal component shows expansile asymmetrical growth, variable, mostly poor maturation and composed of cells with similar cytologic characteristics to those of the epidermal component. Multiple mitoses are present in dermal melanocytes including 2 mitoses near the deep edge of the tumor. There is an associated dysplastic compound nevus.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree