Adjuvant therapy targets melanoma micrometastases in patients with surgically resected disease that carry a high risk of death from melanoma recurrence. In this setting, adjuvant therapy provides the greatest opportunity for cure before progression into advanced inoperable stages. In randomized clinical trials, interferon-alfa has been shown to have a significant impact on relapse-free survival and, at high dosage, on overall survival compared with observation (E1684) and the GMK vaccine (E1694). This article reviews melanoma adjuvant therapy along with the ongoing and planned clinical trials.
Key points
- •
Adjuvant interferon-alfa is the current standard of care with demonstrable relapse-free survival and overall survival (high-dose regimen) benefit for high-risk melanoma.
- •
New agents like immune checkpoint inhibitors and targeted therapies are being actively investigated.
- •
Therapeutic predictive biomarker studies may further refine patient selection.
Introduction
Malignant melanoma is increasing in incidence at a faster rate than any other malignancy in the United States, where it currently represents the fifth most common cancer in men and the seventh most common cancer in women. In 2014, it is estimated that 76,100 patients will be diagnosed with melanoma in the United States, and about 9710 will die from this disease. Careful surveillance in high-risk individuals, early diagnosis, and prompt surgical removal remain the mainstay of management of operable melanoma. For high-risk melanoma, adjuvant therapy targets micrometastatic disease, which is the source of future mortality from melanoma recurrence and presents an opportunity for curing this disease. Various modalities, including immunologic therapy, chemotherapy, and radiation therapy, have been tested in the adjuvant setting over the past 3 decades.
Clinical Predictors of Risk in Patients with Melanoma
The 2002 American Joint Committee on Cancer (AJCC) tumor, lymph node, and metastasis (TNM) staging system was updated in 2009 and new prognostic factors that have practical implications were added. Stages I and II are grouped as localized melanoma that is restricted to the skin. Stage III is characterized by the presence of lymph node involvement and/or in-transit metastases, whereas stage IV comprises distant metastatic spread.
A vital factor for primary melanoma is the depth of the primary tumor (Breslow tumor thickness). Tumor thickness increases by every millimeter, and the survival rate declines ( Table 1 ). In the presence of ulceration of the primary tumor, survival rates become proportionately lower than nonulcerated melanoma of equivalent T category but are similar to those of patients with a nonulcerated melanoma of the subsequent T category. Increased mitotic rate (at least 1 mitosis/mm 2 ) is strongly correlated with diminished survival rates. It has replaced the Clark level of invasion as a complementary criterion to ulceration for differentiating T1a versus T1b primary tumor.
| T Classification | 10-y Survival (%) |
|---|---|
| T1 = thickness ≤ 1 mm | 92 |
| T2 = thickness 1–2 mm | 80 |
| T3 = thickness 2–4 mm | 63 |
| T4 = thickness >4 mm | 50 |
| T4a = T4 nonulcerated (IIB) | 71 |
| T3b = T3 ulcerated (IIB) | 68 (ulceration despite lesser tumor size affects prognosis) |
| T4b = T4 ulcerated (IIC) | 53 |
Involvement of regional lymph nodes or the presence of intralymphatic (satellite or in-transit) metastasis comprises stage III. The seventh edition of the AJCC staging system has no minimum threshold of lymphatic tumor burden defining the presence of regional nodal metastases. In particular, lymph node tumors of less than 0.2 mm that were ignored in the 2002 staging version were included because minute lymph node deposits (including detection by immunohistochemical staining) are thought to be relevant to recurrence and mortality. For the same T stage, the nodal subclassification N1a (micrometastasis) and N1b (macrometastasis) constitute stage IIIA and stage IIIB, respectively ( Table 2 ). In-transit lymphatic metastases without and with metastatic lymph nodes correspond with N2c and N3, respectively. This population of patients without distant spread of primary melanoma who are at high risk for recurrence and death is 3 times the size of the population with metastatic disease.
| Nodal Staging | 5-y Survival (%) |
|---|---|
| Any N1 (single metastatic node) | 70 |
| Any N2 (2–3 nodes) | 30–50 |
| Any N3 (>4 nodes/matted nodes/in-transit metastases/satellites with metastatic nodes) | 39 |
| IIIA | 78 |
| IIIB | 59 |
| IIIC | 40 |
For advanced disease with metastasis to distant sites with specific attention to number and location, lactate dehydrogenase (LDH) blood levels are key to prognosis. One-year survival of patients with M1c disease (visceral metastases or any distant metastasis with high LDH) is 33%, compared with 62% for M1a melanomas (distant skin, subcutaneous, and lymph node metastases) and 53% for M1b melanomas (lung metastases). Oligometastatic melanoma that is amenable to surgical removal may still have good survival rates if chosen appropriately.
Indications for Adjuvant Therapy
Adjuvant therapy has been tested primarily in AJCC stages IIB, IIC, and III, in which maximal benefit has been proved. These patients have an estimated risk of recurrence that exceeds 30% (ranging from 30% chance of recurrence for IIB to 60% chance of recurrence for IIIC).
Immunotherapy with Interferon-alfa
The type I interferon (IFN) family includes IFN-α, IFN-beta, IFN-epsilon, IFN-kappa, and IFN-omega, whereas IFN-gamma alone constitutes the family of type II IFN. Among the IFNs, IFN-α2 has been the most widely studied clinically.
Mechanism
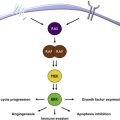
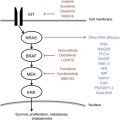
The IFN molecule is thought primarily to induce an immunomodulatory effect and less of a directly cytotoxic or antiangiogenic effect. Research in IFN-α in the neoadjuvant setting has shown significant influence of IFN-α on signal transducer and activator of transcription (STAT) signaling and the histopathologic course of events that take place in the tumor. An influx of dendritic cells (DCs) and T lymphocytes into the tumor tissue was noted. The tumor upregulates STAT3 and there is subsequent elaboration of vascular endothelial growth factor (VEGF), tumor growth factor beta, and interleukin (IL)-10 among other mediators of immune tolerance. IFN-α downregulated STAT3 expression in tumor cells. Also, simultaneous induction of STAT1 in the lymph nodes was observed and this correlated with a reversal in T-cell signaling defects.
IFN-α: Regimens Testing High-dose IFN-α
The impetus to study IFN-α in the adjuvant setting for high-risk resected melanomas was derived from the evidence of activity of IFN-α in the metastatic setting. Table 3 summarizes phase II trials of IFN-α in metastatic melanoma. The first 2 randomized trials that studied the benefits of postsurgical adjuvant therapy for high-risk melanoma were the North Central Cancer Treatment Group (NCCTG) trial and the Eastern Cooperative Group (ECOG) trial E1684. Both trials tested high-dose IFN-α (HDI) (>10 million units [MU]/dose).
| Study Reference | Number of Enrolled Patients (Follow-up) | Therapy and IFN Subspecies | Dose: Treatment Arm (MU/m 2 ) | Schedule: Treatment Arm | ORR | CR | PR |
|---|---|---|---|---|---|---|---|
| Ernstoff, 1983 | 17 | IFN-α2b | 10–100 | 5 d/wk × 1 mo | NA | NA | 2 |
| Creagan, 1984 | 23 | IFN-α2a | 50 | Thrice weekly × 12 wk | 20 | 1 | 5 |
| Creagan, 1985 | 350 | IFN-α2a + cimetidine | 50 | Thrice weekly × 12 wk | 23 | 0 | 8 |
| Creagan, 1984 | 31 | IFN-α2a | 12 | Thrice weekly × 12 wk | 23 | 3 | 4 |
| Legha, 1987 | 62 | IFN-α2a | First arm: escalating (3–36 × 10 6 U/d) | First arm: daily during induction followed by thrice weekly | First: 12.9% | First: 0 | First: 9.7% |
| Second arm: fixed dose (18 × 10 6 U/d) | Second arm: thrice weekly | Second: 16.1% | Second: 0 | Second: 6.5% | |||
| Hersey, 1985 | 200 | IFN-α2a | 15–50 | Thrice weekly | 10 | 2 | 0 |
| Dorval, 1986 | 22 | IFN-α2b | 10 | Thrice weekly | 24 | 2 | 4 |
| Neefe, 1990 | 97 | IFN-α2a | Escalating: 3 to 36 × 10 6 U | Daily for 10 d then 70 d total | 8 | 6 | 2 |
| Coates, 1986 | 15 | IFN-α2a | 20 | 5 d/wk every 2 wk | 0 | 0 | 0 |
The ECOG E1684 trial was initiated in 1984 using a regimen consisting of intravenous (IV) HDI at 20 MU/m 2 for 5 consecutive days a week for 4 weeks as the induction phase followed by subcutaneous (SC) administration at 10 MU/m 2 thrice weekly for 48 weeks as maintenance. At a median follow-up of 6.9 years, 287 patients were studied in total, showing a statistically significant difference in relapse-free survival (RFS) and overall survival (OS) in favor of HDI compared with the observation arm. The estimated 5-year RFS in the treatment arm was 37% (95% confidence interval [CI], 30%–46%) versus 26% (95% CI, 19%–34%) in the control group. The 5-year OS was 46% (95% CI, 39%–55%) versus 37% (95% CI, 30%–46%) in the treatment and observation arms, respectively. The greatest impact on survival was observed in patients with clinically node-negative but pathologically positive nodes (N1 disease). The outcomes of this trial led to regulatory approval by the United States Food and Drug Administration (FDA) in 1995.
IFN-α: Other ECOG and Intergroup Trials Testing HDI
When weighed against these survival benefits, the toxicity profile of HDI as observed in E1684, with a 67% incidence for grade 3 toxicity, 9% incidence for grade 4 toxicity, and 2 early therapy-related hepatotoxic deaths, raised concerns over patients’ endurance and adherence to this regimen. These concerns motivated investigators to study other forms or regimens that varied by dose level, route of administration, or duration of IFN-α therapy. Table 4 lists the phase III adjuvant trials of IFN-α conducted in high-risk melanoma.
| Study Reference | Number of Patients Eligible for Analysis | TNM Stage | Therapy and IFN Subspecies | IFN Dose and Schedule: Treatment Arm | Median Follow-up at Time of Reporting (y) | DFS | OS | % Node Positive |
|---|---|---|---|---|---|---|---|---|
| High Dose | ||||||||
| NCCTG 83-7052 Creagan et al | 262 | II–III (T2–T4N0M0/T any N+M0) | IFN-α2a vs observation | IM 20 MU/m 2 thrice weekly for 4 mo | 6.1 | NS | NS | 61 |
| ECOG E1684 Kirkwood et al | 287 | II–III (T4N0M0/T any N+M0) | IFN-α2b: high dose (HDI) vs observation | IV 20 MU/m 2 5 d a week for 4 wk and then SC 10MU/m 2 3 d a week for 48 wk | 6.9, 12.1 | S | S (S at 6.9 y. NS at 12.1 y) | 89 |
| ECOG E1690 Kirkwood et al | 642 | II–III (T4N0M0/T any N+M0) | IFN-α2b: high dose (HDI) vs low dose vs observation | High dose: IV 20 MU/m 2 5 d a week for 4 wk and then SC 10 MU/m 2 3 d a week for 48 wk Low dose: SC 3 MU/m 2 2 d a week for 2 y | 4.3, 6.6 | S | NS | 75 |
| ECOG E1694 Kirkwood et al | 774 | II–III (T4N0M0/T any N+M0) | IFN-α2b: high dose (HDI) vs GMK vaccine | IV 20 MU/m 2 5 d a week for 4 wk and then SC 10 MU/m 2 2 d a week for 48 wk | 1.3, 2.1 | S | S | 77 |
| Italian Melanoma Intergroup Chiarion-Sileni et al | 330 | III (T any N1–N3M0) | Intensified IFN-α2b (IHDI) every other month vs IFN-α2b high dose (HDI) for 1 y | IHDI: IV 20 MU/m 2 5 d/wk for 4 wk every other month for 4 cycles Standard HDI: IV 20 MU/m 2 5 d/wk for 4 wk then SC 10 MU/m 2 3 d/wk for 48 wk | 5.0 | NS | NS | 100 |
| Intermediate Dose | ||||||||
| EORTC 18952 Eggermont et al | 1388 | II–III (T4N0M0/T any N+M0) | IFN-α2b for 1 y vs 2 y vs observation | IV 10 MU 5 d a week for 4 wk and then SC 10 MU 3 d a week for 1 y Or SC 5 MU 3 d a week for 2 y | 4.65 | NS | NS | 74 |
| EORTC 18991 Eggermont et al | 1256 | III (T any N+M0) | PEG–IFN-α2b vs observation | SC 6 μg/kg/wk for 8 wk and then SC 3 μg/kg/wk for 5 y | 3.8 | S | NS | 100 |
| Low Dose | ||||||||
| Austrian Melanoma Cooperative Group Pehamberger et al | 311 | II (T2–T4N0M0) | IFN-α2a vs observation | SC 3 MU 7 d a week for 3 wk and then SC 3 MU 3 d a week for 1 y | 3.4 (mean) | S | NS | 0 |
| French Melanoma Cooperative Group Grob et al | 499 | II (T2–T4N0M0) | IFN-α2a vs observation | SC 3 MU 3 d a week for 3 y | >3 | 0.74 (HR), S | 0.70 (HR), S | 0 |
| WHO-16 Cascinelli et al | 444 | III (T any N+M0) | IFN-α2a vs observation | SC 3 MU 3 d a week for 3 y | 7.3 | NS | NS | 100 |
| Scottish Melanoma Cooperative Group Cameron et al | 96 | II–III (T3–T4N0M0/T any N+M0) | IFN-α2a vs observation | SC 3 MU 3 d a week for 6 mo | 6.5 | NS | NS | NA |
| EORTC 18871/DKG-80 Kleeberg et al | 728 | II–III (T3–T4N0M0/T any N+M0) | IFN-α2b vs IFN-γ vs ISCADOR M vs observation | IFN-α2b: SC 1 MU every other day for 12 mo | 8.2 | NS | NS | 58 |
| IFN-γ: SC 0.2 mg every other day for 12 mo | NS | NS | ||||||
| ISCADOR M® | NS | NS | ||||||
| UKCCCR/AIM-HIGH Hancock et al | 674 | II–III (T3–T4N0M0/T any N+M0) | IFN-α2a vs observation | SC 3 MU 3 d a week for 2 y | 3.1 | NS | NS | 70 |
| DeCOG Hauschild et al | 840 | III (T3 any N+M0) | IFN-α2a for 18 mo (A) vs 3 y (B) | SC 3 MU 3 d a week for 18 mo vs 3 y | 4.3 | NS | NS | 18 |
| DeCOG Garbe et al | 441 | III (T any N+M0) | IFN-α2a (A) vs IFN-α2a + DTIC (B) vs observation (C) | SC 3 MU 3 d a week for 24 mo (A) vs | 3.9 | S | S | 100 |
| SC 3 MU 3 d a week for 24 mo + DTIC 850 mg/m 2 every 4–8 wk for 24 mo (B) vs | NS | NS | ||||||
E1690
This trial conducted by the ECOG and the US Intergroup followed suit using the E1684 HDI regimen and compared its benefit with a low-dose regimen of IFN-α2b (LDI) at 3 MU SC thrice weekly for 2 years and a third arm consisting of patients who were observed without therapy (Obs). Accrual of patients in E1690 was completed between 1991 and 1995, and at 4.3 years median follow-up the 5-year estimated RFS rates were 44% for HDI, 40% for LDI, and 35% for the Obs arm. The effect of HDI on RFS alone reached significance ( P = .03). Neither HDI nor LDI established OS benefit compared with Obs (52% high dose vs 53% low dose vs 55% observation). However, improved OS of the E1690 Obs arm was notable compared with E1684 Obs arm (median 6 years vs 2.8 years). Although, unlike E1684, E1690 did not require elective lymph node dissection, a retrospective analysis showed evidence of crossover from the observation arm at regional nodal recurrence to IFN-α salvage therapy that may have affected the survival analysis in E1690.
E1694
This trial conducted by the US Intergroup compared HDI with a ganglioside vaccine, which was considered the optimal vaccine candidate at the time. The GMK vaccine consisted of purified ganglioside GM2 coupled to keyhole limpet hemocyanin (KLH) and combined with the QS-21 adjuvant. It was hypothesized that vaccination kindled antibodies against GM2 capable of exclusively attaching to GM2 and knocking off malignant melanocytes in vitro via complement or antibody-based cell-medicated cytotoxicity. HDI was superior compared with GMK with improved RFS (hazard ratio [HR], 1.47; P = .001) and OS (HR, 1.52; P = .009).
E2696
E2696 was an ECOG-led, randomized, phase II that recruited 107 patients with surgically resected stage IIB, III, and IV disease that was conducted between 1998 and 2000. The intent was to study the anti-GM2 antibody response to GMK vaccine in the presence versus absence of IFN. The study compared 3 arms: arm A (GMK plus concurrent HDI), arm B (GMK plus sequential HDI), and arm C (GMK alone). The combined approach reduced the risk of recurrence compared with GMK alone (HR 1.96 for C vs B and HR 1.75 for C vs A).
IFN-α: Other Doses, Routes and Durations Tested
The search for less toxic and more efficacious regimens led to multiple trials using other dosing ranges, routes of administration, durations of therapy, and formulations. Although the Sunbelt Melanoma Trial studied lymph node dissection (LND) versus LND plus standard HDI, the Italian Melanoma Group trial studied a shorter duration of a more intense course of IFN than the standard regimen, with no statistically significant differences seen.
Hellenic trial
The Hellenic Oncology Group intended to test the hypothesis that the IV induction phase of the HDI regimen was the most important part of the regimen and was sufficient in exerting the therapeutic impact of HDI in high-risk melanoma. In the phase III He 13A/98 study, patients were randomized between 1998 and 2004 to a modified induction phase of 15 MU/m 2 HDI only versus the same induction phase followed by a modified maintenance phase of 10 MU (not per square meter) thrice weekly for a year. With 182 patients per arm, and a median follow-up of 5.25 years, the analysis in 2009 revealed no statistically significant difference in either median RFS or OS. However, the study was criticized for the modified regimen used and the small sample size to allow it to show a clinically significant difference.
E1697
US Intergroup study E1697 tested a similar hypothesis among patients with resectable intermediate risk melanoma (≥T3 or any thickness with microscopic nodal disease N1a–N2a). Between 1998 and 2010, the study recruited 1150 patients and randomized them to either 4 weeks of HDI (20 MU/m 2 /d for 5 days weekly) or observation. In 2010, a third interim analysis deemed the study futile and the study was closed. When presented to American Society of Clinical Oncology (ASCO) in 2011, the study reported no impact on either RFS or OS with this 4-week regimen. The results of this trial supported the E1684 HDI 1-year regimen as the standard for high-risk melanoma.
Other trials have investigated less intensive regimens for the dosing of IFN-α. These trials included low-doses (1 MU SC every other day) tested in the European Organization for Research and Treatment of Cancer (EORTC) 18871 trial (stage IIB, IIIA), low doses (≤3 MU SC thrice weekly) tested in the World Health Organization (WHO) Melanoma Trial 16 (stage III), E1690 (T4, N1), the UKCCCR AIM-HIGH trial (stage IIB/III), a Scottish trial (stage IIB, III), and the 2010 German Dermatologic Cooperative Group (DeCOG) study (T3 any N). Intermediate dose regimens (5–10 MU/m 2 ) were tested in the EORTC 18952 (T4 N1-2) and EORTC 18991 (TxN1) studies. Although these trials showed benefit in RFS for the IFN arms, this impact was lost with time. Support for this observation also comes from the French multicenter trial, which indicated that the effect of IFN-α on RFS was lost on cessation of treatment.
EORTC 18952
This trial enrolled 1388 patients with stage IIB/III disease to 4 weeks of induction with 10 MU IV 5 times a week, followed by one of 2 maintenance regimens” SC 10 MU 3 days a week for 1 year versus SC 5 MU 3 days a week for 2 years. The third arm was an observation control arm. The study was conducted from 1996 to 2000. At 4.65 years median follow-up, the results showed a statistically insignificant 7.2% increase in distant metastasis-free interval (47% vs 43% and 40% respectively) and a 5.4% increase in OS (53% in the 2-year arm compared with 48% each in the 1-year and observation arms). The increase in OS was observed only in patients treated for 25 months with 5 MU IFN-α2b and not in those treated for 13 months with 10 MU IFN-α2b. These results suggest that the duration of therapy might be more important than the dose.
DeCOG
In 2008, a randomized phase III DeCOG trial studied a combination of LDI/dacarbazine (DTIC) versus LDI alone. Analysis at 4 years median follow-up revealed that the low-dose IFN group showed an improvement in disease-free survival (DFS) (HR, 0.69) and OS (HR, 0.62). However, the trial was designed to find whether DTIC adds any benefit to IFN-α and not whether LDI was superior to observation. These results do not match the earlier trials that tested LDI (ie, the Austrian Melanoma Cooperative Group trial and French Melanoma Cooperative Group trial ), which showed no OS benefit for LDI. Unlike in North America, LDI therapy has been approved as an adjuvant therapy for stage II patients by the European Medicines Agency in Europe. Regional differences exist in Europe in the adjuvant use of IFN. High-dose IFN regimens are not used in Europe as commonly as in the United States.
Pegylated IFN
EORTC 18991
Pegylated (peg) IFN-α as tested in this trial achieved regulatory approval for use as adjuvant therapy for high-risk melanoma with lymph node metastases. The covalent bonding of the IFN molecule with a polyethylene glycol moiety results in sustained absorption and longer half-life. The EORTC 18991 trial studied the efficacy and safety of peg–IFN-α2b versus observation among 1256 patients recruited from 2000 to 2002 with resected AJCC stage III melanoma. The regimen comprised an induction dose of peg-IFN SC 6 μg/kg a week for 8 weeks followed by maintenance dose of once-weekly SC injections at 3 μg/kg for up to 5 years. At 7.6 years median follow-up, the group released data that showed an improved RFS in the treatment arm (HR, 0.87; 95% CI, 0.76–1.00; P = .05) with no difference in OS/distant metastases free survival (DMFS) between observation and treatment groups. Subset analysis indicated that subjects with microscopic nodal metastasis and ulcerated primary tumor had the greatest benefit in terms of RFS, OS, and DMFS. During the study, peg-IFN was discontinued for toxicity in 37% of patients.
IFN-α: Meta-analyses
At least 4 different systematic reviews and meta-analyses on adjuvant therapy have been published from 2002 to 2010. The largest was a 2010 meta-analysis from Mocellin and colleagues that included randomized controlled trials published between 1990 and 2008 covering 8122 patients, of whom 4362 subjects had received IFN-α. In 12 of the 14 studies included, IFN-α was tested against observation, and 17 different comparisons were established. In subgroup analysis, no specific regimen, dosing, formulation, study design, or staging provided any difference in overall HR estimates. Four of 14 comparators revealed a statistically significant OS benefit with IFN-α. The review concluded that adjuvant IFN-α therapy showed a statistically significant 18% risk reduction for recurrence (HR, 0.82; 95% CI, 0.77–0.87; P <.001) and 11% risk reduction for death (HR, 0.89; 95% CI, 0.83–0.96; P = .002).
Predictors of Benefit and Prognostic Markers
The consistent but modest beneficial effect of adjuvant IFN-α has been well shown, but it also comes at the expense of significant toxicity and cost. Hence, there is a need to focus treatment on patients who are most likely to benefit from adjuvant IFN-α therapy, as was noted in some of the trials. After the Wheatley meta-analysis and later a subset analysis of EORTC 18991 revealed specific benefit among patients with ulcerated primary tumors, some focus has been placed on targeting such patient groups in clinical trials including the ongoing EORTC 18081. This trial will study adjuvant peg-IFN for 2 years versus observation in patients with an ulcerated primary cutaneous melanoma with T(2–4)b, N0, M0 melanoma.
Gogas and colleagues in 2006 reported a prospectively validated analysis of autoimmunity as a biomarker associated with IFN-α benefit. Also, our group at the University of Pittsburgh and ECOG studied these data from the E2696 and E1694 trials to further understand the newly found association between autoimmune-related side effects and improved outcomes with IFN-α. A landmark analysis of E1694 revealed a trend toward a survival advantage associated with HDI-induced autoimmunity in stage III patients treated with HDI. In both trials, the presence of autoantibodies in sera of patients was significantly more frequent in the HDI arm compared with the vaccine arm. However, the development of autoimmunity occurs over a period of up to 1 year and therefore cannot be used as a baseline or early on-treatment predictor of IFN-α therapeutic benefit. We are currently testing the immunogenic predictors of autoimmunity associated with IFN-α in the context of the E1697 trial as potential predictors of IFN-α therapeutic benefits. The expression of methylthioadenosine phosphorylase, which plays a significant role in the activity of STAT1, has shown an association with improved OS and RFS, as noted in a retrospective study from Meyer and colleagues. Accumulating data reinforce the importance of the relative balance of phosphoSTAT1 (pSTAT1)/pSTAT3 in the tumor microenvironment. Serum markers of interest include S100B, melanoma-inhibiting activity, and tumor-associated antigen 90 immune complex (TA90IC). Tarhini and colleagues, showed that a high or increasing serum level of S100B is an independent prognostic marker of risk for mortality in patients with high-risk disease, as tested in the context of the E1694 trial.
Introduction
Malignant melanoma is increasing in incidence at a faster rate than any other malignancy in the United States, where it currently represents the fifth most common cancer in men and the seventh most common cancer in women. In 2014, it is estimated that 76,100 patients will be diagnosed with melanoma in the United States, and about 9710 will die from this disease. Careful surveillance in high-risk individuals, early diagnosis, and prompt surgical removal remain the mainstay of management of operable melanoma. For high-risk melanoma, adjuvant therapy targets micrometastatic disease, which is the source of future mortality from melanoma recurrence and presents an opportunity for curing this disease. Various modalities, including immunologic therapy, chemotherapy, and radiation therapy, have been tested in the adjuvant setting over the past 3 decades.
Clinical Predictors of Risk in Patients with Melanoma
The 2002 American Joint Committee on Cancer (AJCC) tumor, lymph node, and metastasis (TNM) staging system was updated in 2009 and new prognostic factors that have practical implications were added. Stages I and II are grouped as localized melanoma that is restricted to the skin. Stage III is characterized by the presence of lymph node involvement and/or in-transit metastases, whereas stage IV comprises distant metastatic spread.
A vital factor for primary melanoma is the depth of the primary tumor (Breslow tumor thickness). Tumor thickness increases by every millimeter, and the survival rate declines ( Table 1 ). In the presence of ulceration of the primary tumor, survival rates become proportionately lower than nonulcerated melanoma of equivalent T category but are similar to those of patients with a nonulcerated melanoma of the subsequent T category. Increased mitotic rate (at least 1 mitosis/mm 2 ) is strongly correlated with diminished survival rates. It has replaced the Clark level of invasion as a complementary criterion to ulceration for differentiating T1a versus T1b primary tumor.
| T Classification | 10-y Survival (%) |
|---|---|
| T1 = thickness ≤ 1 mm | 92 |
| T2 = thickness 1–2 mm | 80 |
| T3 = thickness 2–4 mm | 63 |
| T4 = thickness >4 mm | 50 |
| T4a = T4 nonulcerated (IIB) | 71 |
| T3b = T3 ulcerated (IIB) | 68 (ulceration despite lesser tumor size affects prognosis) |
| T4b = T4 ulcerated (IIC) | 53 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree