67 Melanoma
Melanoma Epidemiology
In the United States, the melanoma incidence rate has risen faster than for any other cancer and is nearly double that of liver, lung, or prostate cancer. Between 1950 and 2000, the incidence of melanoma among Caucasians increased by 619%, an average of 4.3% per year, although the rate of increase has declined in the last decade, suggesting a potential positive impact of primary prevention campaigns.1 Rapid increases in melanoma incidence have been attributed to environmental risk factors and increased sun exposure behaviors, but controversy exists over whether these incidence trends are simply a result of expanded skin screening,2 biopsy rates,3 and reporting of low-risk, biologically indolent tumors to cancer registries.4 Persistent high proportions of thicker melanomas in U.S. Surveillance Epidemiology and End Results (SEER) data and worldwide would seem to mitigate the impact of improved screening and suggest the increased burden of melanoma is a global reality.5
Invasive cutaneous melanoma occured in an estimated 68,720 Americans in 2009 (39,080 men and 29,640 men); an additional 53,120 or more cases of melanoma in situ were diagnosed.6 While the median age of diagnosis is 53 years, melanoma is the most common cancer in women and second only to breast cancer in women in their early 30s.
Melanoma is currently the fifth most common cancer in men and the sixth in women, but it is not a major cause of death in either gender.6 Case-based fatality has declined sharply for melanoma over the past several decades as a result of earlier detection of thinner melanomas that lack or have lower biological potential to metastasize. Current 5-year survival rates exceed 90% in the United States, Australia, and Sweden and are uniformly higher in developed countries than developing countries, where relative survival may be as low as 40%.7,8
Although melanoma accounts for roughly 4% of all skin cancers, it is responsible for more than 75% of skin cancer deaths.6 The most striking differences in melanoma mortality occur in individuals aged 50 or older, who should be a primary target for early detection and screening efforts. Of the estimated 8650 deaths from melanoma in the United States in 2009, more than 64% occured in men (5550 men compared to 3100 women).9 Analysis of SEER data has demonstrated a disproportionate burden of melanoma deaths among middle-aged and older Caucasian men. Between 1973 and 2002, mortality rates rose 64% in U.S. Caucasian men aged 55 through 64 years and 130% (8.6 to 19.8 per 100,000) in men 65 or older.10 This is in contrast to middle-aged women of the same age group, who experienced markedly lower mortality increases (15%), and to both younger men and women (aged 20 to 54 years), who showed declining mortality rates over this period (11% and 23%, respectively).10
Etiology
The process by which normal melanocytes transform into melanoma cells is poorly understood but likely involves progressive genetic mutations that alter cell proliferation, differentiation, and death and impact cellular susceptibility to the carcinogenic effects of ultraviolet radiation.11 It has been estimated that 65% of melanomas in Caucasian populations worldwide is in some way attributable to sun exposure.12 The highest melanoma incidence rates worldwide are observed in fair-complexioned individuals residing in sunny climates, lending credence to the role of excessive ultraviolet radiation (UVR) in melanoma pathogenesis.
A complex relationship among environmental exposures, phenotypic characteristics, and genotypic traits is believed to predispose an individual to melanoma. Multiple risk factors have been identified, including both natural and artificial UVR, other extrinsic factors (occupational exposures, ionizing radiation, and potential dietary deficiencies), host immunosuppression, presence of atypical nevi, and genetic factors.1 Evidence-based analyses support the hypothesis that melanoma risk is affected primarily by intermittent, intense sun exposure, particularly in childhood or adolescence.13,14 In contrast, chronic occupational exposure does not appear to confer increased risk, except perhaps for the more UV-related melanoma subtypes (lentigo maligna and lentigo maligna melanoma).
Ultraviolet B radiation (UVB; wavelengths 290 to 320 nanometers) appears more closely linked with development of melanoma than ultraviolet A (UVA; wavelengths 320 to 400 nanometers).14 This is supported by statistically higher incidence of melanoma in equatorial regions where UVB radiation is most intense compared to latitudes farther from the equator. UVA intensity varies less across latitudes.15,16
Excessive exposure to UV radiation in childhood appears to be particularly important in that 25% and 80% of lifetime sun exposure is estimated to occur before age 20.17 Individuals with five or more severe sunburns in childhood or adolescence have an estimated twofold greater risk of developing melanoma.13 Furthermore, the incidence of melanoma is higher among people who move from northern to more equatorial latitudes, particularly if they were children at the time of relocation.18
Commercial tanning beds, which mainly emit UVA light, have been strongly associated with the development of skin cancer, including melanoma.19 Early studies revealed an elevated risk for melanoma with regular sunbed tanning after adjusting for additional risk factors, with higher risk for those who began sunbed tanning earlier in life.20 A subsequent systematic review by the International Agency for Research on Cancer of pooled data from 19 international studies demonstrated a 15% higher melanoma risk in both men and women who had ever used tanning beds (summary relative risk of 1.15; 95% CI, 1.00-1.31).21 More alarming was the finding of a 75% increased melanoma risk in individuals who first used tanning beds prior to age 35 (summary relative risk 1.75; 95% CI, 1.35-2.26).21 In the United States, adolescent access to indoor tanning is currently regulated in only 25 states, and where regulations exist, compliance is generally low. Of additional concern, businesses actively market their product to minors, which contributes in part to the estimated 35% regular use of indoor tanning facilities by adolescent girls.22 Recent reductions in melanoma incidence in younger individuals (age 20 to 44) may be offset by indoor tanning use in decades to come.
Exposure to oral methoxsalen (psoralen) and UVA radiation (PUVA) is associated with a delayed increase in the risk of melanoma, demonstrating both long latency period and dose-related melanoma risk. In one study, the incidence rate ratio for invasive or in situ melanomas was elevated 15 years following PUVA treatment, increasing to 5.4 by years 16 to 20 and to 9.3 beyond 20 years of follow-up.23 Individuals with a history of nonmelanoma (keratinocytic) skin cancer (basal cell and squamous cell carcinomas) have an estimated threefold increased melanoma risk, likely related to common etiologic factors such as excessive UVR and sun-sensitive phenotype.24,25
Exposure to occupational hazards has been examined in a number of studies focusing on polychlorinated biphenyls (PCBs), petroleum products, ionizing radiation, and selenium. Although initial analyses showed patterns of increased incidence, no statistically significant occupational risk factors were found after adjustment for known risk factors such as nevus count and sun exposure.26,27
The majority of studies addressing the role of dietary factors (e.g., antioxidants, retinoids, vitamin C, and vitamin E) have not shown a consistent effect on melanoma incidence. Case-control studies of diets rich in vitamin D and carotenoids and low in alcohol suggest an associated reduction in melanoma risk.28 Multiple studies addressing the role of oral contraceptives or hormone replacement therapy have found no evidence of increased risk for melanoma.29
Cutaneous melanoma occurs more commonly in immunosuppressed patients, such as organ transplant recipients, and immunosuppression appears to be related to worse prognosis (increased recurrence and death) in patients with higher-risk tumors. In patients with melanoma treated prior to transplantation, recurrences were estimated at 19% in one series, and usually within 5 years.30 Waiting at least 5 years between the treatment of thicker melanomas and transplantation may reduce the risk of recurrent disease resulting from transplant-associated immunosuppression.31 It remains controversial whether an increased incidence of melanoma occurs in HIV-infected populations, although recent studies have suggested an increased risk of melanoma in immunosuppressed cohorts.32
The presence of clinical atypical nevi (CAN), also termed dysplastic nevi, is the most important clinical risk factor for melanoma. Compared with the general population, patients with CAN have a 3- to 20-fold elevated risk of developing malignant melanoma, and risk increases with the number of CAN present and for those who also have a personal or family history of melanoma.33,34 Importantly, only about one-third of all melanomas are estimated to arise from nevus precursors, including congenital, common, and dysplastic nevi, and the estimated percentage of melanomas that arise within atypical nevi is in the range of 10% to 30%.35,36 Since CAN are best considered as risk markers for melanoma rather than precursor lesions, their widespread removal is generally not indicated. However, patients with CAN constitute one of the highest risk groups for melanoma and should receive routine dermatologic surveillance and education regarding melanoma warning signs as well as importance of regular skin self-examination to enhance early detection.
Studies suggesting that high levels of sun exposure are inversely associated with death from melanoma seem to corroborate the novel concept of at least two pathways of melanoma pathogenesis.37 This theory asserts that melanomas in chronically sun-exposed skin (i.e., head and neck) may be more prevalent in older patients with higher cumulative doses of sun exposure and low nevus count, whereas intermittent UV exposure may be more closely linked to truncal melanoma and high nevus count in younger patients.38
This dichotomy in mole phenotype, melanoma site, age of onset, and UVR pattern is supported on a molecular level as well. Recent data have shown that differences in frequency of BRAF or NRAS mutations are also related to patterns of sun exposure, with BRAF mutations more common in intermittently UV-exposed skin compared with chronically sun-damaged skin or relatively unexposed skin, such as acral or mucosal sites, which demonstrate KIT mutations more frequently.39 Further elucidation of this etiologic, phenotypic, and genetic heterogeneity will not only improve the public health messages for melanoma prevention but also result in more effective targeted therapies.
Genetics
The majority of melanomas are not hereditary in nature, and only 5% to 10% estimated to be due to a familial predisposition.40 Germline mutations in the cyclin-dependent kinase inhibitor 2A (CDKN2A/p16)/cyclin-dependent kinase 4 (CDK4) cell-cycle and tumor-suppressor gene axis and in the melanocortin-1 receptor (MCR1) pigmentation-associated gene have been associated with melanoma pathogenesis, as have somatic mutations in the PTEN and BRAF genes.41,42 Melanoma in association with other tumors, including pancreatic, breast, and brain tumors, may be associated with germline mutations in CDKN2A or potentially BRCA2.43 Additional melanoma risk occurs in CDKN2A mutation carriers who express variants of the MCR1 gene, which is associated with red hair, fair skin, and freckling.44
About 40% of hereditary melanoma is attributed to germline mutations in CDKNA/p16, making it the most important melanoma susceptibility gene identified to date.45 Commercial availability for CDKN2A/p16 genetic testing has heightened the need to develop evidence-based guidelines for genetic assessment in appropriate individuals. While a positive or negative p16 mutation result does not often change the need for ongoing dermatologic surveillance in patients with strong family or personal history of melanoma and/or atypical mole phenotype, it may affect the need for surveillance for pancreatic cancer in individuals with familial predisposition to develop this cancer.43
Pathology
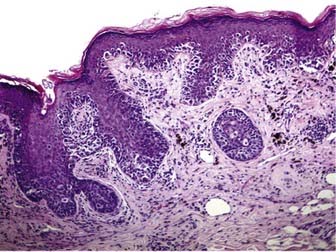
Melanoma in situ is characterized histologically by a poorly circumscribed proliferation of markedly atypical melanocytes, which may be nested or solitary along the basal layer of the epidermis, and which may or may not exhibit pagetoid upward scatter, depending on histologic subtype.46 Superficial spreading melanoma has an in situ (radial growth) phase characterized by increased numbers of intraepithelial melanocytes, which are large and atypical, arranged haphazardly at the dermoepidermal junction, and show upward (pagetoid) migration (Fig. 67-1). Melanomas in situ of the lentigo maligna and acral lentiginous subtypes demonstrate predominant “lentiginous” growth at the dermoepidermal junction (e.g., solitary atypical melanocytes at the epidermal basal layer) and less intraepidermal pagetoid scatter of cells, although lentigo maligna may exhibit nested aggregates of melanocytes and histologic features similar to those observed in dysplastic nevi.47 Lateral intraepidermal extension of melanoma cells occurs in all subtypes except nodular melanoma, which exhibits a prominent dermal nodular aggregate of atypical melanocytes and a relatively small in situ component, typically limited to the area directly over or just beyond the invasive dermal component.46
Dermal invasion of cutaneous melanoma confers metastatic potential, with the greatest risk believed to occur in the setting of a vertical growth (tumorigenic) phase.48,49 Tumorigenicity is characterized by a distinct population of melanoma cells with evidence of proliferation (mitoses, MIB-1 staining) and nuclear pleomorphism within the dermis and possibly involving the subcutaneous fat. Failure of melanocyte maturation and dispersion as the tumor extends downward into the dermis is characteristic of invasive melanoma (Fig. 67-2).
Tumor thickness, as defined by the Breslow depth, is the most important histologic determinant of prognosis and is measured vertically in millimeters from the top of the granular layer (or base of superficial ulceration) to the deepest point of tumor involvement. Increased tumor thickness confers a higher metastatic potential and a poorer prognosis.50,51 Analysis of the American Joint Committee on Cancer (AJCC) collaborative melanoma database for both the 6th and 7th edition of the AJCC Cancer Staging Manual melanoma staging guidelines (published in 2001 and 2009, respectively) showed that histologic ulceration of the primary tumor is the next most important determinant of patient prognosis, and its presence upstages individuals to the next more advanced AJCC stage.52,53 The most recent worldwide analysis of the AJCC melanoma staging database also confirmed that elevated mitotic rate (≥1 mitosis/mm2) confers worse overall survival, and it will now be used to classify thin melanomas (≤1 mm) as T1b. While not incorporated into current 2010 AJCC microstaging for melanoma, lymphovascular invasion of the primary tumor has also been reported to have both prognostic value and potential correlation with sentinel lymph node positivity.54
Clinical Presentation
Primary cutaneous melanoma may occur anywhere on the body, although in the United States, it is most commonly diagnosed on the lower extremities and back in women and the trunk in men.55 Certain melanoma subtypes, such as lentigo maligna melanoma and acral lentiginous melanoma, occur in characteristic locations as discussed later. In addition to clinical warning signs such as asymmetry, border irregularity, color variegation, and diameter larger than 6 mm (the “ABCD” criteria), surface features such as elevation and ulceration may be useful in predicting whether melanoma is early or advanced.56 Recognition of evolving (E) features of a skin lesion is an additional criterion to assist in early detection.57
Histogenetic Subtypes
Controversy persists regarding the validity of subtyping melanoma, but recent evidence of divergent pathways of melanomagenesis according to anatomic location, sun exposure, nevus count, age of onset, and various mutations (e.g., BRAF, KIT) suggest that distinct phenotypic and genotypic patterns of melanoma exist.38,39 Most multivariate analysis have shown that histogenetic type is not an independent prognostic variable for survival after controlling for tumor thickness; however, there is little doubt that the nodular subtype accounts for most newly diagnosed deep primaries, and that it is a subtype that may elude early detection.5 With the exception of nodular melanoma, all growth patterns are characterized by a preceding in situ (intraepithelial) phase that lacks the biological potential to metastasize and may last from months to years before invasion occurs. As such, melanoma in situ is completely cured following excisional surgery.51
Superficial Spreading Melanoma
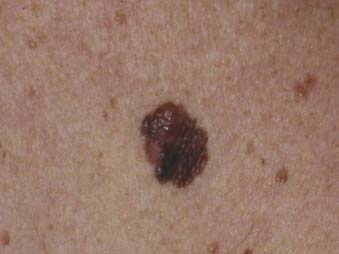
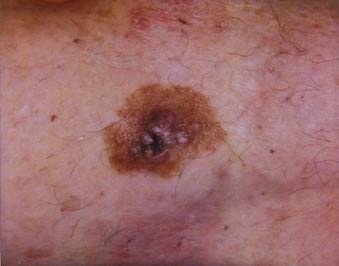
Superficial spreading melanoma is the most common subtype of melanoma, accounting for about 70% of all cases, particularly between the ages of 30 and 50.58 It occurs most frequently on the upper back of men and women as well as the lower extremities of women. The clinical lesion typically shows irregular, asymmetric borders with color variegation (e.g., black, blue, or pink), and its size is generally larger than 6 to 8 mm (Fig. 67-3). Superficial spreading melanoma characteristically exhibits some or all of the ABCD criteria, and widespread use of these clinical warning signs over the past 2 decades is believed to have contributed to significantly thinner melanomas of this subtype being diagnosed.5
Nodular Melanoma
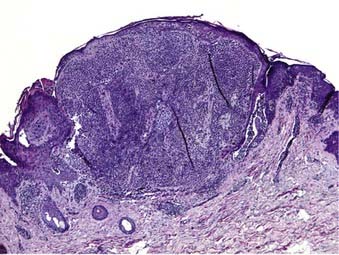
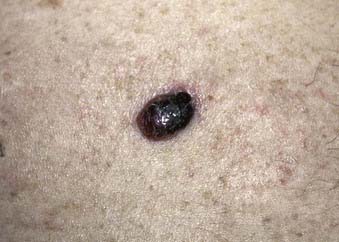
Nodular melanoma is the second most common subtype of melanoma, accounting for 15% to 30% of all types and is more common in men than women.58 The median age of diagnosis is 53 years; however, thicker nodular melanomas are associated with older age.5 Like superficial spreading melanoma, legs and trunk are the most frequent sites of involvement. Clinically, the lesion presents as a raised, dark brown to black papule or nodule, and ulceration and bleeding are common (Fig. 67-4). Rapid growth over weeks to months is a hallmark of nodular melanoma, typically resulting in greater thickness at diagnosis compared to other melanoma subtypes. Despite its seemingly more aggressive clinical behavior, nodular melanoma has a prognosis similar to superficial spreading melanoma when matched for tumor thickness.59
Lentigo Maligna Melanoma
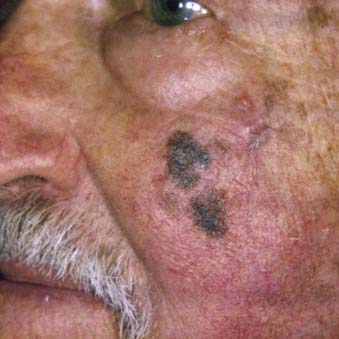
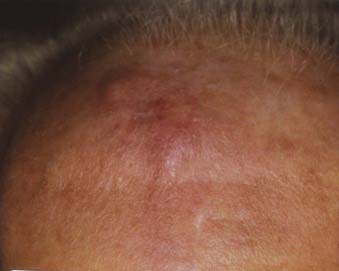
Lentigo maligna melanoma accounts for 4% to 15% of cutaneous melanoma and is typically located on the head, neck, and arms (sun damaged skin) of elderly, fair-skinned individuals (mean age 65).58 Recent characterization of melanoma subtype incidence has suggested increasing rates of both in situ and invasive lentigo maligna subtypes, particularly in individuals older than 50.60 Like nonmelanoma (keratinocytic) skin cancers (e.g., basal cell and squamous cell carcinomas), lentigo maligna melanoma is linked to cumulative rather than intermittent sun exposure. The face is the most common site of involvement, particularly the nose and cheeks. The precursor in situ lesion, lentigo maligna, is usually present for over 5 to 20 years and often attains large size (>1 to 3 cm diameter) before progression to lentigo maligna melanoma occurs. Lentigo maligna appears as a tan to brown macule or patch with variation in pigment or areas of regression that appear hypopigmented (Fig. 67-5). Only 5% to 8% of lentigo maligna are estimated to evolve to invasive melanoma, and this event is characterized by nodule development within the flat precursor lesion (Fig. 67-6).61
Acral Lentiginous Melanoma
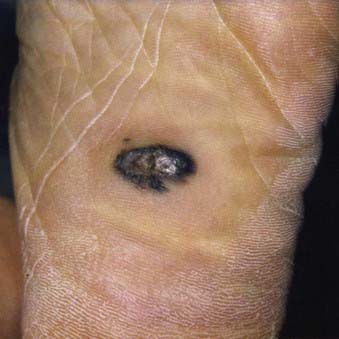
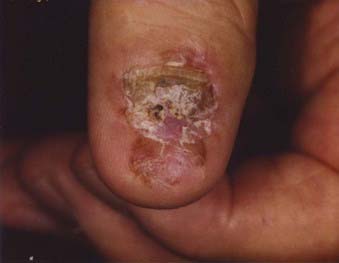
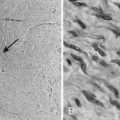
Acral lentiginous melanoma is the least common subtype in the United States, representing only 2% to 8% of melanoma in Caucasians, although it accounts for 29% to 72% of melanoma in dark-complexioned individuals (African Americans, Asians, and Hispanics).58,62 It typically occurs on the palms or soles or beneath the nail plate (subungual variant). A small percentage of superficial spreading and nodular melanoma may also be located acrally. Patients are generally middle-aged to elderly, with an average onset in the sixth decade. Irregular pigmentation, large size (≥3 cm diameter), and plantar location are characteristic features of acral lentiginous melanoma (Fig. 67-7). Subungual melanoma occurs most commonly on the great toe or thumb and is characterized by rapid onset of diffuse nail discoloration or a longitudinal pigmented band within the nail plate, although nail dystrophy may also occur. The additional presence of pigmentation extending into the proximal or lateral nail folds (Hutchinson’s sign) strongly suggests subungual melanoma and warrants biopsy of the nail matrix from which these melanomas arise (Fig. 67-8). Subungual melanoma may be confused with a benign junctional nevus, pyogenic granuloma, infectious process (bacterial or fungal), or subungual hematoma. If subungual hematoma is suspected, a history of trauma should be elicited and the lesion followed to ensure resolution with continued growth of the nail plate.
Rare Melanoma Variants
Unusual subtypes of primary melanoma include desmoplastic/neurotropic melanoma, mucosal (lentiginous) melanoma, malignant blue nevus, melanoma arising in giant congenital nevus, and melanoma of soft parts (clear cell sarcoma). Together, these variants account for less than 5% of primary melanomas.58 Desmoplastic melanoma may occur in association with macular, lentigo maligna-type pigmentation or present de novo as a firm, amelanotic nodule or scar (Fig. 67-9). Desmoplastic melanoma predominantly occurs on sun-exposed areas of the head and neck, with a mean age between 60 and 65 years.63 Lack of pigmentation and clinical features more suggestive of keratinocytic skin cancer may result in delay in detection and thicker tumors at diagnosis. Desmoplastic melanoma frequently exhibits perineural extension and has a predilection for local recurrence, somewhat analogous to a skin sarcoma. Wide excisional margins (≥2 cm) and adjuvant radiation therapy are frequently recommended for improved local control of this uncommon melanoma subtype.
Diagnostic and Staging Studies
The most important aspects of the initial workup for patients diagnosed with primary cutaneous melanoma are a careful history, review of systems, and physical examination.64 Published data have shown that baseline and surveillance laboratory studies (e.g., lactate dehydrogenase [LDH] level, liver function tests), chest radiography (CXR), and other imaging studies (computed tomography [CT], positron emission tomography [PET], bone scintigraphy, magnetic resonance imaging [MRI]) are not typically beneficial for stage I/II (cutaneous) melanoma patients who have no signs or symptoms of metastasis.65–68
National Comprehensive Cancer Network (NCCN) practice guidelines support the concept that most melanoma recurrences are diagnosed clinically. Current recommendations advise against the practice of obtaining baseline studies in patients with asymptomatic cutaneous melanoma, including melanoma in situ (stage 0) and stages I and II invasive primary melanoma. The 2009 guidelines emphasize that imaging (CT scan, PET, MRI) should be obtained to evaluate specific signs or symptoms suspicious for disease recurrence. Routine surveillance laboratory tests and radiologic imaging to screen for asymptomatic metastasis are not recommended for stage IA to stage IIA melanoma (invasive primaries ≤4 mm depth). However, surveillance CXR, CT and/or PET/CT may be considered to screen for metastasis in asymptomatic patients with stage IIB (>4 mm) to stage IV disease, although there is not uniform consensus of the NCCN melanoma panel for this recommendation (category 2B).69
While abnormal laboratory tests are rarely the sole indicator of metastatic disease, elevated serum LDH levels are associated with worse survival in patients with stage IV (distant) disease and have thus been incorporated since 2002 into the AJCC melanoma staging guidelines.70 Serum S-100 protein levels may also be useful as a tumor marker in patients with metastatic disease, but this practice is not widely employed in the United States.71
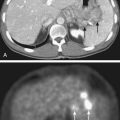
Sentinel lymph node biopsy (SLNB) is typically performed for pathologic staging of the regional nodal basin(s) for primary tumors 1 mm depth or more and may be considered in thinner melanomas when certain high-risk histologic features (e.g., ulceration, high mitotic rate, lymphovascular invasion) are present.69 A metastatic workup (CT, PET or PET/CT, brain MRI) should be initiated if physical findings or symptoms suggest disease recurrence, or if the patient has documented sentinel lymph node metastasis, although the low tumor burden in the setting of microscopic regional nodal metastasis is generally not associated with synchronous detection of visceral metastasis.
Imaging Studies
Primary Cutaneous Melanoma
As previously noted, imaging studies such as CT scans, MRI, PET, ultrasonography, and bone scans have an extremely low yield in asymptomatic patients with primary cutaneous melanoma (AJCC stages I and II) and are generally not indicated. However, maintaining a low threshold for obtaining symptom-directed tests is important for melanoma surveillance. Baseline metastatic staging for melanoma patients with primary tumors greater than 4 mm in depth may include CXR but remains optional in the absence of signs or symptoms of metastatic disease.69
Sentinel node status (positive or negative) is the most important prognostic factor for recurrence and is the most powerful predictor of survival in patients with melanoma.72 Current AJCC melanoma staging and NCCN clinical practice guidelines advocate pathologic staging of the regional lymph nodes for cutaneous melanoma of greater than 1 mm depth (T1b to T4b and for thinner (T1a) melanomas with high-risk histologic features, along with microstaging of the primary melanoma, as the most complete means of staging.69,73
Metastatic Melanoma
Melanoma surveillance for stage III (regional nodal) or stage IV (distant) disease often involves imaging with CT, PET, combined PET/CT, and/or MRI and is generally indicated for determining the extent of disease in patients with recurrent melanoma to guide management and assess whether surgical resection is appropriate. Whole body PET has shown increased sensitivity, specificity, and accuracy for detection of metastasis compared with CT (chest, abdomen, and pelvis), although combined PET/CT provides vital anatomic information not available with PET alone.74,75 As with cutaneous melanoma, NCCN guidelines promote the use of more extensive imaging studies to follow up suspicious signs and symptoms, but surveillance CT scans for asymptomatic patients with stage IIB and IIC high-risk primaries or stage III/IV melanoma may be considered at the clinician’s discretion.69
Melanoma Staging System
The AJCC staging system for melanoma underwent a major modification in 2002, following an evidence-based review of prognostic information collected on 17,600 melanoma patients from 13 international cooperative groups and major cancer centers.52,70 This analysis identified novel factors for incorporation into the staging classification of melanoma for the T, N, and M categories. As indicated previously, in the T category, primary tumor thickness and ulceration were found to be the most important prognostic factors of those analyzed by the AJCC staging committee in patients with localized, cutaneous melanoma. As a result, these two factors have been incorporated into the T classification, with tumor thickness being the predominant stratification factor, with cutoffs of 1.0 mm, 2.0 mm, and 4.0 mm. Thus, the T1 category includes melanomas 1.0 mm thick or less; T2, 1.01 to 2.0 mm; T3, 2.01 to 4.0 mm; and T4 greater than 4.0 mm thick. Each T category is further classified by the absence (a) or presence (b) of ulceration, which denotes a histologic loss of epidermal continuity overlying the primary melanoma. In the prognostic analysis presented, the presence of ulceration within a given tumor thickness stratum upstaged the tumor to the next stratum without ulceration.
The 2010 version of the AJCC staging classification has largely abandoned the use of the Clark level, which measures the anatomic depth of penetration into the skin by the melanoma and was incorporated into the prior version of the staging classification.54 In 2002, Clark level was retained only in thin, T1 tumors in which the presence of either ulceration or a deep tumor (i.e., Clark level IV or V) would upstage the tumor to T1b. Effective 2010, the AJCC melanoma staging system will instead incorporate mitotic rate 1/mm2 or more to upstage T1a to T1b tumors. The T classification described five substages with worsening survival based on tumor thickness and histologic ulceration: stage IA, with an estimated 97% 5-year survival; stage IB, with 91% to 94% 5-year survival; stage IIA, with 79% to 82% 5-year survival; stage IIB, with 68% to 71% 5-year survival; and stage IIC, with estimated 53% 5-year survival.
In the nodal (N) category, the 2002 AJCC system defined nodal involvement as presence of metastatic melanoma within the lymph node on routine hematoxylin and eosin staining.70 Thus, patients with only immunohistochemical positive staining of the lymph node or those with positive detection of tumor markers using quantitative polymerase chain reaction were not included in the node-positive category. However, the 2009 iteration will now include sentinel lymph node metastasis detected by immunohistochemical staining alone.53
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree