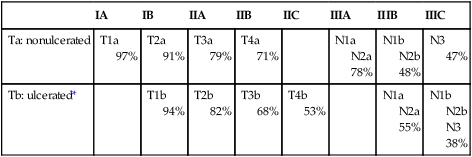
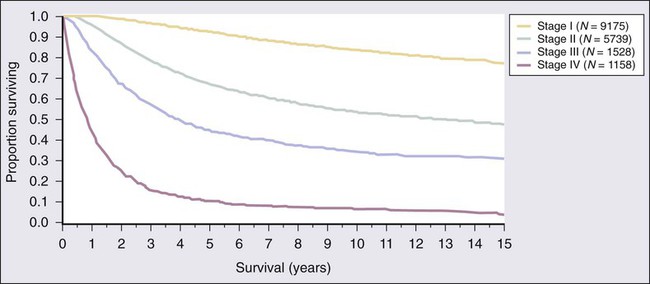
Tara C. Gangadhar, Leslie A. Fecher, Chris J. Miller, Giorgos Karakousis, Robert Vonderheide, George Xu and Lynn M. Schuchter • The incidence of melanoma has increased dramatically during the past few decades. • Approximately 76,250 new cases of invasive melanoma are diagnosed each year in the United States, and it is estimated that 1 in 36 men and 1 in 55 women in the United States will be diagnosed with melanoma in their lifetime. • The risk of melanoma is strongly related to exposure to ultraviolet irradiation; people with fair hair and skin, a tendency to burn, and numerous benign or atypical nevi are also at increased risk. • Approximately 50% of melanomas have a somatic activating mutation in BRAF. • The important pathologic features of the primary lesion are thickness (in millimeters), the presence or absence of histologic ulceration, and the mitotic rate, all of which have prognostic value. • A full-thickness biopsy should be performed for any suspicious, new, or changing lesion; an excisional biopsied is preferred. • The current American Joint Committee on Cancer staging system includes the thickness and presence or absence of ulceration of the primary tumor, the number of positive nodes, whether the nodes are microscopically or macroscopically positive, and whether distant disease is present. • The extent of radiologic staging evaluation depends on the risk of recurrence. In general, computed tomography or positron emission tomography/computed tomography imaging can be considered for patients with stage III melanoma. • Surgery is the primary treatment for melanoma. All primary melanomas require a wide local excision for local control. • The margins of wide excision are determined by the thickness of the primary lesion. • Sentinel lymph node mapping is a useful staging procedure for melanoma and provides prognostic information. • A sentinel lymph node biopsy should be offered to patients with melanomas >1 mm in thickness or melanomas ≤1 mm that are associated with either ulceration or a mitotic rate >1 mitosis/mm2 and can be considered for patients with other high-risk features (e.g., size >0.75 mm, lymphovascular invasion, or Clark level IV). • The current standard of care is to offer patients with known nodal involvement a regional completion lymph node dissection. • Adjuvant therapy with high-dose interferon alfa can be offered to patients with high-risk resected disease (nodal metastases or a primary tumor >4 mm). • Observation or participation in a clinical trial are additional options for patients with stage III or high-risk stage II melanoma. Treatment of Metastatic Disease • The median survival for patients with melanoma after distant metastatic disease has been identified is approximately 9 to 12 months. • Treatment options include molecularly targeted therapy, immune therapy, cytotoxic chemotherapy, and participation in clinical trials. • All patients with advanced melanoma should have their tumor tissue assessed for the presence or absence of the BRAF V600 E mutation. The presence of the mutation is predictive of response to targeted therapy with BRAF inhibitors. • Immune therapy options include CTLA-4 inhibition with ipilimumab, high-dose interleukin-2, and clinical trials. • Cytotoxic chemotherapy options include dacarbazine-based or temozolomide-based therapy. • Additional supportive and palliative care options should be considered for all patients; brain metastases are managed with surgery and/or radiation therapy, depending on the size and number of lesions. The incidence of melanoma rose by an average of 2.9% each year between 1985 and 2009. In 2012, an estimated 76,250 men and women in the United States were diagnosed with invasive melanoma.1 The lifetime risk for developing melanoma is estimated to be 1 in 36 for men and 1 in 55 for women. Melanoma is the fifth most common type of cancer in men and the sixth most common type of cancer in women. Mortality rates for melanoma have also increased, although at a slower rate than the increase in incidence. An estimated 9180 men and women will die of melanoma in the United States in 2012. The majority of melanomas are associated with exposure to UVR, which is the major environmental factor contributing to melanoma risk. Both ultraviolet B (UVB) and ultraviolet A (UVA) radiation are carcinogenic and can induce melanoma. The role of sun and UVR in the development of melanoma is supported by several epidemiological studies demonstrating an increased incidence of melanoma in people who live close to the equator or at higher altitudes and in persons who report increased exposure to UVR. A prospective study has also confirmed the association between UVR exposure and increased melanoma risk.2 The risk from UVR varies according to intensity (i.e., sunburn vs. no sunburn), frequency, and age at the time of exposure. Intermittent, intense UVR exposure and sunburns increase melanoma risk; this risk increases as the number of sunburns and intermittent intensive UVR exposures accumulate. Several studies emphasize the increased risk associated with UVR exposure during childhood, but melanoma risk also increases with increasing number of sunburns during all life periods, not just during childhood. The most common anatomic locations of melanoma for men (the trunk) and women (the legs) correspond to areas more commonly exposed to intermittent, intense UVR. Chronic UVR exposure also increases risk for melanoma; a history of chronic exposure (as opposed to intense intermittent exposure) is often noted for melanomas of the upper limbs, head, and neck. Exposure to artificial UVR also increases melanoma risk. Therapy with artificial UVR is used most commonly for the treatment of inflammatory disorders, such as psoriasis or atopic dermatitis. Therapy with oral 8-methoxypsoralen-UV-A increases the risk of melanoma. The recreational use of tanning beds, which emit mostly UVA radiation, is a more common source of exposure to artificial UVR and is a major public health problem. Certain phenotypes such as blond or red hair have been associated with an increased risk of melanoma. The “red hair color” (RHC) phenotype increases the risk for melanoma when combined with exposure to UVR. The RHC phenotype is characterized by fair pigmentation (i.e., fair skin, red hair, and freckles) and by sun sensitivity (i.e., poor tanning response and solar lentigines). Variants of the human melanocortin-1 receptor gene (MC1R) are associated with the RHC phenotype. MC1R signals through a key pathway within melanocytes via the microphthalmia-associated transcription factor (MITF) to control the pigmentary phenotype (skin color) by regulating the relative proportion of eumelanin (brown/black pigment) and pheomelanin (red/yellow pigment) in the skin and hair. Germline variants in MC1R that disrupt signaling are present in approximately 80% of persons with RHC. Variants of MC1R cause a quantitative shift of melanin synthesis from eumelanin to pheomelanin, resulting in the RHC phenotype and increased risk for melanoma as a result of a relative lack of eumelanin. Patients with MCIR variants are at increased risk for developing melanoma, particularly the variants most strongly associated with RHC (termed “R variant”).3 The presence of increased numbers of nevi, large nevi, and clinically atypical nevi are risk factors for melanoma. Clinically, atypical nevi have a size of 5 mm or larger and have at least two of the following characteristics: variable pigmentation, irregular, asymmetric outline, and indistinct borders. Increasing numbers of dysplastic nevi increase the risk for melanoma. The presence of 10 or more clinically atypical (also known as “dysplastic”) nevi confers a twelvefold increased risk for developing melanoma. In the absence of atypical nevi, increased numbers of nevi still confer a two- to fourfold increased risk for melanoma.4 Although the presence of nondysplastic and dysplastic nevi predicts an increased risk for melanoma, only a small percentage of nevi progress to melanoma. Approximately 20% to 30% of melanomas arise in conjunction with a melanocytic nevus. Most melanomas arise de novo from clinically normal skin. Large congenital melanocytic nevi (>20 cm in diameter), particularly those arising on the torso in a “bathing trunk” distribution, have an estimated risk of 2.5% to 5% of degeneration to malignant melanoma. The development of melanoma in small (1.4 cm or less) and intermediate (1.5 to 19.9 cm) congenital melanocytic nevi is rare. A family history of melanoma increases one’s risk for melanoma. The risk for melanoma is 2.62 times greater when a parent has had a melanoma and is 2.94 times greater when a sibling has had a melanoma.5 These increased risks apply when first-degree family members have sporadic melanomas, which constitute 90% of all melanomas. Fewer than 10% of patients present with true familial melanomas. In families with autosomal dominantly inherited susceptibility mutations, melanomas develop at an early age and multiple primary melanomas develop; multiple cases of melanoma occur in several family members across different generations on one side of the family, and, in some cases, other associated cancers occur as well. Several genetic loci determine susceptibility to melanoma, with the most important of these being cyclin-dependent kinase 4 (CDK4) and the cyclin-dependent kinase inhibitor 2A gene (p16/CDKN2A), located on chromosome 9p21. The CDKN2A gene encodes two proteins, p16 and p14ARF, which are cell-cycle inhibitors.6 Both proteins are potent tumor suppressors. Both CDNKN2A and CDK4 are highly penetrant susceptibility genes and result in the majority of familial melanomas. The CDKN2A mutation is present in up to 40% of families with three or more cases of melanoma, whereas CDK4 mutations are present in far fewer families. The risk of developing cutaneous melanoma in a person who is a CDKN2A mutation carrier is between 30% by age 5 years and 67% by age 80 years; this risk varies by geographic location and is higher is sunnier regions. The same risk factors that influence the incidence of melanoma in the general population (i.e., total nevus counts, presence of dysplastic nevi, and sunburn) also increase penetrance in CDKN2A mutation carriers. Up to 10% of patients with multiple primary melanomas have also been identified as having a CDKN2A mutation. Pancreatic cancer is also seen in melanoma-prone families with CDKN2A germline mutations.7 In contrast to CDKN2A and CDK4, which are high-penetrance melanoma predisposition genes, heritable mutations in the melanocortin-1 receptor (MC1R) gene have lower penetrance. MC1R mutation is commonly associated with a red hair phenotype, but it confers an increased risk of developing melanoma even in the absence of red hair.3,8 Some familial melanoma occurs in the setting of the familial atypical multiple primary mole and melanoma syndrome, also called the dysplastic nevus syndrome.8 A family history of melanoma in multiple first-degree relatives and younger age at diagnosis are important features of this syndrome. Families who present with the familial atypical multiple primary mole and melanoma syndrome may also have CDKN2A mutations. Other heritable disorders that predispose patients to melanoma include xeroderma pigmentosum, a rare inherited disorder in which DNA repair mechanisms are compromised, resulting in an extremely high rate of skin cancers, including cutaneous and conjunctival melanomas, Li-Fraumeni syndrome, BRCA2, or familial retinoblastoma. Cell-cycle regulatory proteins are required for the precise regulation of cell growth and division and therefore are critical targets in the malignant transformation of all cells. The observation of frequent deletions in the 9p21 locus in familial primary melanomas led to the identification of the CDNK2A tumor-suppressor gene in familial melanoma.9,10 The CDNK2A gene encodes an inhibitor of the cyclin-dependent kinases, CDK4 and CDK6, which leads to cell-cycle arrest at the G1 phase. If p16 is not expressed, CDK4/CDK6 may phosphorylate the retinoblastoma protein Rb and inactivate its tumor suppressor function. Expression of CDNK2A is silenced in sporadic melanomas via multiple mechanisms, including epigenetic inactivation through promoter methylation CDK4 amplification and cyclin D gene amplification. Loss of p16 expression is associated with disease progression and a poor prognosis.11 The mitogen-activated protein kinase (MAPK) signal transduction pathway, which is composed of RAS, BRAF, MEK (mitogen-activated protein kinase), and extracellular signal-regulated kinase (ERK) signaling, regulates cell growth, survival, and invasion. This pathway is aberrantly activated in many cancers. RAS, a membrane-bound guanosine triphosphatase, is frequently mutated in human cancer but has proven challenging to target therapeutically. RAS mutations are not common in melanoma; they are described in approximately 20% of melanomas and most often in NRAS.12,13 Somatic activating mutations of the serine/threonine kinase BRAF were first described in 59% of melanoma cell lines and in six of nine primary melanomas.14 The majority of BRAF mutations (80%) are V600E, located in exon 15 and characterized by a single amino acid substitution of valine for glutamic acid at codon 600 that renders the kinase constitutively active. A subsequent study of genome-wide alterations in DNA copy number and BRAF and NRAS mutational status in primary human melanomas demonstrated a correlation between genetic alterations and four unique melanoma subtypes based on anatomic site and UV exposure: mucosal melanoma, acral melanoma, and cutaneous melanomas with and without chronic sun-damaged (CSD) skin (defined by presence of solar elastosis). Approximately 80% of cutaneous melanomas on non-CSD skin (intense intermittent sun exposure) have mutations in either BRAF or NRAS (59% and 22%, respectively). BRAF mutations are less common in melanomas of CSD skin and rare in melanomas of mucosal, uveal, and other noncutaneous origin. V600K BRAF mutations constitute approximately 20% of V600 BRAF mutant melanomas.15 In addition to V600E and V600K mutations, less common BRAF mutations such as V600D or V600R, K601, and L597 have been reported. Germline mutations in BRAF have not been identified. The phosphatidylinositol-3-kinase (PI3K) signaling pathway, which is activated by cell surface receptor tyrosine kinases or G-protein coupled receptors, including RAS, is important in cell growth, proliferation, motility, and survival and is frequently altered in cancers. Akt supports cell growth and survival via activation of the mammalian target of rapamycin (mTOR), as well as other activities. PTEN (phosphatase and tensin homolog deleted on chromosome 10) is a tumor suppressor that promotes cell cycle arrest and apoptosis and negatively regulates Akt and the PI3K pathway. In melanoma, the most common alterations of the PI3K pathway include NRAS mutations, functional loss of PTEN, and/or Akt overexpression. Alterations of PTEN that result in functional loss include allelic loss/deletion, mutations, and epigenetic silencing. Mutations in PIK3CA, AKT1, and AKT3 are rare. Increased phosphorylated-Akt overexpression, with or without PTEN loss, has been reported in brain metastases compared with extra–central nervous system (CNS) metastases.16 KIT (also known as CD117), a receptor tyrosine kinase, plays a critical role in melanocyte development, proliferation, differentiation, migration, and survival. KIT activation leads to activation of downstream targets, including the MAPK, PI3K, and signal transducer and activator of transcription (STAT) signaling pathways and microphthalmia-associated transcription factor (MITF). KIT alterations (mutations and/or copy number increases) have been identified in 28% of cutaneous CSD melanomas, 36% of acral melanomas, and 39% of mucosal melanomas. Of these alterations, activating mutations were present in 17% of cutaneous, 11% of acral, and 21% of mucosal melanomas. Of note, KIT abnormalities were not identified in any non-CSD cutaneous melanomas, which commonly possess NRAS/BRAF mutations.17 MITF, a member of the MiT transcription factor family, is required for normal melanocyte development and plays a role in cell cycle progression, motility, and survival. MITF also plays a major role in pigment production, where its expression is induced when alpha-MSH binds MC1R and, in turn, stimulates synthesis of melanin. MITF has been identified as an oncogene in melanoma with amplification demonstrated in melanoma cell lines, as well as in 10% to 20% of human cutaneous primary melanomas and melanoma metastases, but it is absent in benign nevi.18 Dysregulated MITF acts as an oncogene through altered target gene expression including CDK2, BCL2, MET, CDKN2A, HIF1alpha, and others. Given its interaction with HIF1alpha, the MITF gene has been sequenced in patients with both renal cell carcinoma and melanoma, with identification of a novel germline mutation, MITF E318K, which results in impaired SUMOylation (a posttranslation modification) of the MITF protein and altered target gene regulation.19,20 The process of apoptosis, or programmed cell death, is critical to cellular responses to stress and is a major pathway of cell death induced by radiation therapy and traditional chemotherapy. Melanoma cells can be resistant to therapies that have efficacy in other tumor types; this resistance is related, in part, to their ability to evade normal apoptotic signals. The two major apoptotic pathways are the extrinsic pathway, which is induced on activation of cell-membrane–associated death receptors by their associated ligands, and the intrinsic pathway, which is dependent on mitochondrial membrane permeability in response to cellular stress signals. Both pathways result in the activation of caspases that are critical effectors of apoptosis. Melanomas evade both intrinsic and extrinsic apoptotic pathways, which are critical determinants of tumor response to traditional cytotoxic therapies. The cytochrome c-associated factor Apaf-1 is downregulated in advanced melanomas and influences the death response of melanoma cells to cytotoxic agents.21 In addition, Fas and tumor necrosis factor–related apoptosis-inducing ligand (TRAIL) death receptors are downregulated by a variety of mechanisms, leading to impaired activation of the extrinsic apoptotic pathway. Therapeutic strategies aimed at circumventing impaired apoptotic pathways in melanoma are likely to require multiple interventions to ensure the continued activation of effective death pathways, given the variety of resistance mechanisms present in melanoma cells. UVR exposure is the only modifiable risk factor for melanoma. Protection from UVR is the primary strategy to decrease melanoma risk; optimal use of sunscreen and sun protection results in a decreased risk of melanoma.22 Protection from UVR can mitigate one’s risk for skin cancer, regardless of the age at which it is implemented. Standard recommendations to decrease UVR exposure include avoidance of the sun between the peak hours of 10 am to 4 pm, seeking shade whenever possible, covering the skin surface with sun-protective clothing including wide-brimmed hats, long-sleeved shirts, long pants, and sunglasses, and using broad-spectrum sunscreen with a sun protection factor (SPF) of 15 or greater to cover exposed skin surfaces. Regular application of SPF 15+ sunscreen in adults has been demonstrated to decrease melanoma risk in a randomized trial.23 Appropriate type, timing, frequency, and amount of sunscreen applied are important factors in effective primary prevention. The United States Food and Drug Administration (FDA) has legislated regulations for over-the-counter sunscreens (http://www.fda.gov/forconsumers/consumerupdates/ucm258416.htm). Highlights of these new regulations are that sunscreen products that protect against both UVA and UVB radiation will be labeled “broad spectrum” and “SPF 15” (or higher) on the front label; sunscreen products that are not broad spectrum or that are broad spectrum with SPF values from 2 to14 will be labeled with a warning; and the front label must limit water resistance claims to either 40 minutes or 80 minutes, based on standard testing to determine how much time a user can expect to get the declared SPF level of protection while swimming or sweating. Sun-protective clothing guards the skin from UVR more effectively than sunscreen. As opposed to sunscreen, which is frequently applied unevenly and insufficiently, sun-protective clothing provides uniform and constant protection for the areas it covers. The UVR-protective properties of clothing vary according to the thickness, color, and type of fabric. Most regulatory agencies have adopted the UV protection factor as the standard of measurement of UV protection for clothing. Clothing can achieve a UV protection factor >500, which is vastly superior to sunscreen. Barriers to the implementation of sun protection behaviors include discomfort from sun-protective clothing, inconvenience of applying sunscreen, and denial of personal risk for skin cancer. Furthermore, studies indicate that tanning may be an addictive behavior. Recent legislation may help to decrease access of minors to indoor tanning salons; however, modifying exposure to natural UVR remains a challenge. Patients with early-stage disease have an excellent prognosis after complete excision of the melanoma. Strategies to improve detection of early-stage melanoma include regular self-skin examinations and skin examinations by a trained medical practitioner. Self-skin examination, defined as a careful and deliberate self-conducted examination of all areas of the skin for changes in spots or moles, may reduce mortality from melanoma but are performed by only a small percentage of patients24 and are not recommended for prevention in the general public. The majority of melanomas are detected by patients or their partners. Physician recommendation is one of the strongest determinants of self-skin examination and cancer screening. Health care practitioners may increase compliance by strongly recommending regular self-skin examination and by educating patients to examine their skin with confidence. Offering patients full-body photography can improve their ability to diagnose new and changing nevi during self-skin examination. Full-body photography can aid both patients and physicians by providing a fixed reference point to compare new or changing lesions. Although no strong evidence exists that skin cancer screening in the general population by primary care providers or self-examination reduces skin cancer morbidity and mortality, some evidence indicates that screening for melanoma with skin examinations by experienced physicians reduces melanoma mortality because experienced physicians are more likely to detect melanomas at an earlier stage than are patients. Regular screening skin examinations provide an opportunity for physicians to educate patients and their families about melanoma and to increase efforts aimed at primary prevention. Melanoma has been conceptualized as growing first in a radial growth phase with little risk of metastatic behavior. This phase is followed by the vertical growth phase with the capacity for metastasis.25 Different clinical and histologic features are associated with these two phases. Histologically, the radial growth phase refers to a progressive intraepidermal proliferation of melanocytes in which they are largely confined to the epidermis and the superficial dermis, without forming a tumor in the dermis, and without having competence for metastasis. This radial growth phase precedes the vertical growth phase of melanoma in all subtypes except nodular melanoma, which lacks prominent radial growth. Approximately one third of melanomas arise in association with a preexisting nevus. Distinguishing between melanoma and a benign nevus can be challenging, particularly when attempting to distinguish special types of nevi such as a Spitz nevus, cellular blue nevus, combined nevus, or deep penetrating nevus from a melanoma, or when attempting to distinguish a nevus from a nevoid melanoma.26 In these instances, the histologic differences may be subtle, and interpretation by an experienced dermatopathologist is necessary. The clinical presentation of melanoma includes the physical appearance of the pigmented lesion and the history of any change in shape, size, color, or surface. More than 70% of melanomas are associated with an increase in size and change in color of a pigmented lesion. Most patients report having had a preexisting mole at the site of the melanoma. Itching, burning, or pain in a pigmented lesion should increase suspicion, although melanomas are often not associated with local discomfort. Bleeding and ulceration are signs of advanced melanoma. Most melanomas are varying shades of brown, but they may also be black, blue, or pink. The “ABCDEs” for the recognition melanoma are Asymmetry, Border irregularity, Color variegation, Diameter greater than 6 mm, and Evolving characteristics. In patients with numerous pigmented lesions, practitioners can improve detection of melanomas by looking for the “ugly duckling,” that is, the nevus that deviates in size or color from the patient’s general pattern of nevi.27 Full-body photography provides patients and practitioners with a fixed reference of the appearance of the patient’s skin and can facilitate the detection of new or changing lesions. Dermoscopy, which is widely available in dermatology practices, allows examination of skin lesions without obstruction from skin surface reflections. With appropriate training, dermoscopy improves the sensitivity and specificity of melanoma diagnosis among pigmented lesions.28 Most recently, computerized devices that apply diagnostic algorithms to images of pigmented lesions have been used to improve clinical detection of melanomas. Primary cutaneous melanoma has historically been classified into four subtypes on the basis of distinct clinical and histologic features. These subtypes include superficial spreading melanoma (SSM), lentigo maligna melanoma (LMM), nodular melanoma (NM), and acral lentiginous melanoma (ALM) (Fig. 69-1). The classification excludes multiple subtypes of melanoma, such as desmoplastic melanoma (DM), mucosal melanoma, and uveal melanoma. Although histologic subtype does not directly correlate with clinical behavior, recent studies of genotype-phenotype correlation have demonstrated a higher prevalence of BRAF mutation in SSMs and a higher prevalence of KIT mutation in LMMs. Wallace Clark, Jr., initially described an inverse correlation between increasing anatomic levels (I-V) of microinvasion into the dermis or subcutaneous tissue and survival in 1967. Alexander Breslow subsequently established the correlation between tumor thickness in millimeters and survival. Multivariate statistical analysis has identified several features of the primary melanoma that have prognostic significance. These factors have been incorporated in the seventh (2010) edition of the American Joint Committee on Cancer (AJCC) staging system. In a multifactorial analysis of more than 27,000 patients with localized melanoma (either clinically or pathologically), the most powerful and independent prognostic characteristics of the primary melanoma were tumor thickness, ulceration, and mitotic rate for melanomas ≤1 mm.29 Additional clinical factors that have been associated with outcome (but that are not included in the AJCC staging system) are also outlined in the following sections. Tumor mitotic rate is a reflection of the proliferation rate of the primary melanoma. Although mitotic rate is a continuous variable, a threshold of at least 1 mitosis/mm2 has been identified to have the most significant correlation with survival; melanomas with at least 1 mitotic figure/mm2 have a worse prognosis than do those with no mitotic figures. The mitotic rate has been shown in several studies to be an independent factor in predicting both incidence of sentinel node metastases and survival and is now included in the AJCC “T” staging of melanomas ≤1.00 mm in thickness only.30,31 Pathologists begin the mitotic count with the most active tumor focus; this “hot spot” technique is the recommended method of assessment. Adjacent fields are inspected until a total of 1 mm2 has been examined. The mitotic rate should be reported as “mitoses/mm2.” Several other histologic features of the primary melanoma have prognostic importance but are not included in the current AJCC “T” staging. The presence of tumor-infiltrating lymphocytes (TILs) is associated with a more favorable prognosis. Melanomas should be routinely assessed for lymphocytic infiltration in the vertical growth phase and classified as brisk, nonbrisk, or absent with regard to TILs. TILs are termed brisk if they infiltrate the entire invasive component diffusely or across the base of the VGP. TILs are reported as absent if no lymphocytes are present or if they are present but do not infiltrate the tumor. When lymphocytes infiltrate the melanoma only focally, the term “nonbrisk” is used. The absence of TILs is predictive of a higher risk sentinel lymph node (SLN) metastasis in patients undergoing SLN biopsy (SLNB) for primary cutaneous melanoma but is not predictive of survival.32 Microsatellites are a relatively uncommon feature in melanoma and are defined as any discontinuous nest of metastatic melanoma cells >0.05 mm in diameter that is clearly separated by normal dermis (not fibrosis or inflammation) from the main invasive component of melanoma by a distance of at least 0.3 mm. The presence or absence of microsatellites should be routinely reported because it confers a worse prognosis and is incorporated into the AJCC “N” stage. Lymphovascular invasion is defined by the presence of melanoma cell(s) in the lymphovascular channel. Lymphovascular invasion in the primary melanoma is associated with a higher risk of lymph node metastasis but is not an independent predictor of survival for melanoma in general.33,34 The anatomic site of the primary melanoma has been reported to correlate with survival, with trunk and head and neck sites having a worse outcome than extremity sites; anatomic site is not included in the current AJCC staging system. Although older patients have thicker melanomas and a higher incidence of ulcerated melanomas, age (especially age greater than 60 years) has been reported as an independent adverse prognostic factor, even after multivariate adjustment for other factors.35,36 Finally, men have a worse prognosis compared with women who have a similar melanoma presentation and stage; this finding that has been consistent across many studies. The 7th edition of the AJCC staging system was adopted in 2009 and is based on a study of patient outcomes from the AJCC Melanoma Database, which consisted of a total of 38,918 patients with melanoma, including 7972 patients with stage IV melanoma.29 The data were merged from prospective databases of patients from 17 major medical centers, stand-alone cancer centers, and cancer cooperative groups. Box 69-1 and Tables 69-1 and 69-2 list the current melanoma TNM categories and the stage groupings, respectively. The 15-year survival curves for patients with stage I to IV melanoma are shown in Figure 69-2.The distinction between clinical and pathologic staging is worth noting. Table 69-1 American Joint Committee on Cancer Pathologic Stage Grouping* *Note that the stage groupings involve upstaging to account for melanoma ulceration, where thinner melanomas with ulceration are grouped with the next greatest T substage for nonulcerated melanomas. From American Joint Committee on Cancer. Cancer staging manual. 7th ed. New York: Springer-Verlag; 2010. Table 69-2 American Joint Committee on Cancer 5-year Survival Rates of Pathologically Staged Patients *The presence of tumor ulceration of a primary melanoma (designated Tb) causes upstaging by one substage compared with a nonulcerated melanoma (designated Ta). From American Joint Committee on Cancer. Cancer staging manual. 7th ed. New York: Springer-Verlag; 2010.
Melanoma
Epidemiology
Risk Factors for Melanoma
Environmental Risk Factors—Ultraviolet Radiation/Sun Exposure
Phenotype
Presence of Nevi and/or Atypical Nevi
Family History
Etiology and Biological Characteristics
CDNK2A
RAS, RAF, and the Mitogen-Activated Protein Kinase Pathway
Phosphatidylinositol-3-Kinase
KIT
MITF
Apoptotic Pathways
Prevention and Early Detection
Primary Prevention
Secondary Prevention
Pathology
Melanoma Histopathology
Clinical Manifestations, Patient Evaluation, and Staging
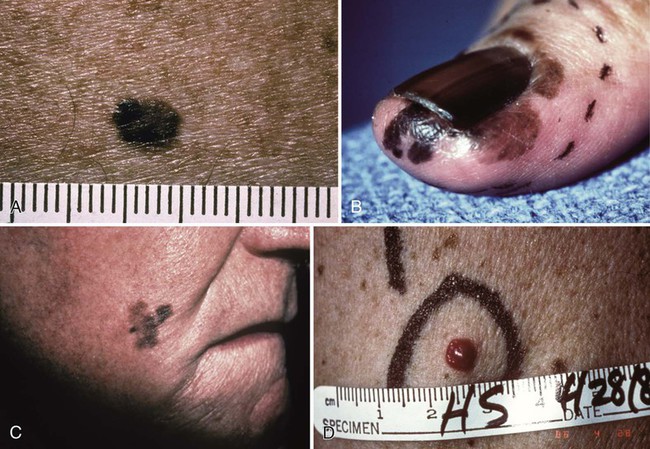
Clinical Presentation
Staging
Biopsy Technique
Prognostic Factors and Microstaging
Dermal Mitotic Rate
Staging Classification
Stage
Grouping
Stage 0
TisN0M0
Stage IA
T1aN0M0
Stage IB
T1bN0M0
T2aN0M0
Stage IIA
T2bN0M0
T3aN0M0
Stage IIB
T3bN0M0
T4aN0M0
Stage IIC
T4bN0M0
Stage IIIA
T1–4aN1aM0
T1–4aN2aM0
Stage IIIB
T1–4bN1aM0
T1–4bN2aM0
T1–4aN1bM0
T1–4aN2bM0
T1–4a/bN2cM0
Stage IIIC
T1–4bN1bM0
T1–4bN2bM0
Any T N3M0
Any T, any N, M1
IA
IB
IIA
IIB
IIC
IIIA
IIIB
IIIC
Ta: nonulcerated
T1a
97%
T2a
91%
T3a
79%
T4a
71%
N1a
N2a
78%
N1b
N2b
48%
N3
47%
Tb: ulcerated*
T1b
94%
T2b
82%
T3b
68%
T4b
53%
N1a
N2a
55%
N1b
N2b
N3
38%