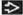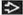(T) Primary Tumor | Adapted from 7th edition AJCC Staging Forms. | |
TNM | Definitions | |
TX | Primary tumor cannot be assessed (e.g., curettaged or severely regressed melanoma) | |
T0 | No evidence of primary tumor | |
Tis | Melanoma in situ | |
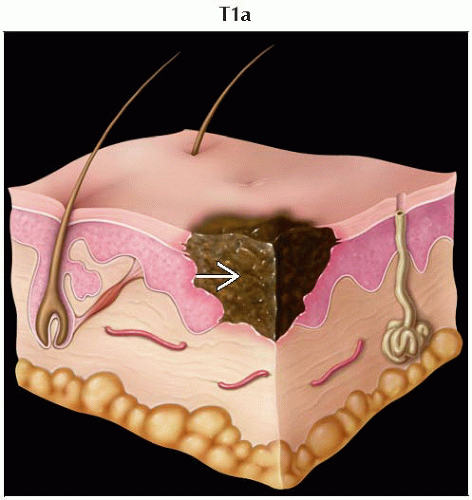
T1 | Melanomas ≤ 1.0 mm in thickness | |
T1a | Melanomas ≤ 1.0 mm in thickness without ulceration and mitosis < 1/mm2 | |
T1b | Melanomas ≤ 1.0 mm in thickness with ulceration or mitoses ≥ 1/mm1 | |
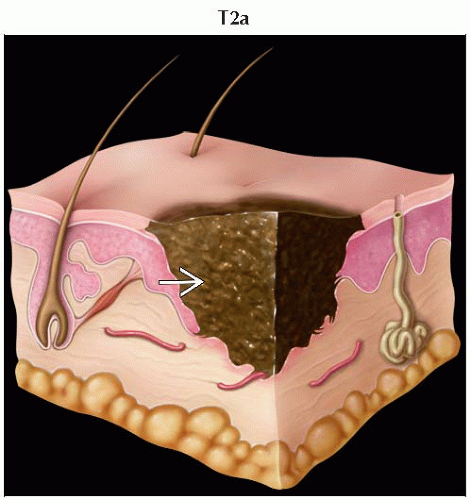
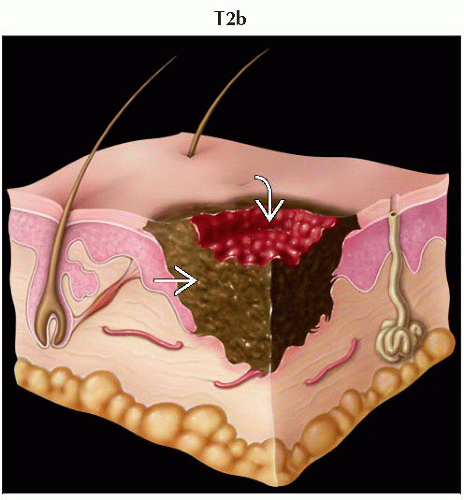
T2 | Melanomas 1.01-2.0 mm | |
T2a | Melanomas 1.01-2.0 mm without ulceration | |
T2b | Melanomas 1.01-2.0 mm with ulceration | |
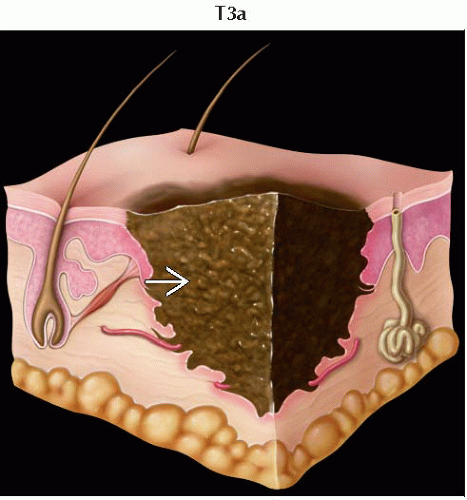
T3 | Melanomas 2.01-4.0 mm | |
T3a | Melanomas 2.01-4.0 mm without ulceration | |
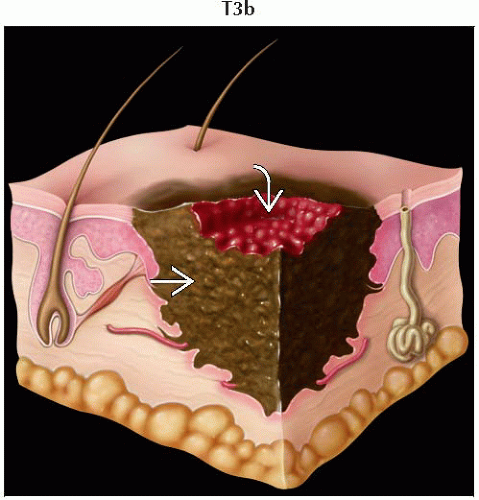
T3b | Melanomas 2.01-4.0 mm with ulceration | |
T4 | Melanomas > 4.0 mm | |
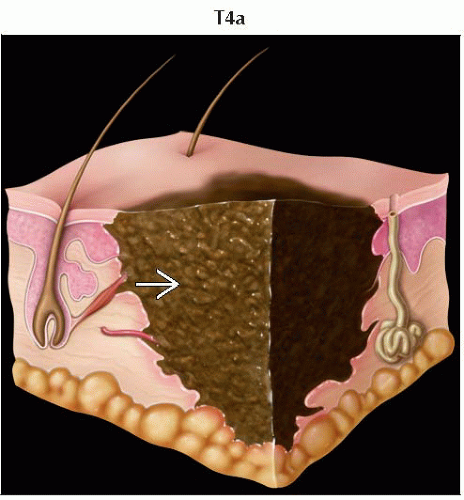
T4a | Melanomas > 4.0 mm without ulceration | |
T4b | Melanomas > 4.0 mm with ulceration | |
(N) Regional Lymph Nodes | ||
NX | Patients in whom regional nodes cannot be assessed (e.g., previously removed for another reason) | |
N0 | No regional metastases detected | |
N1 | 1 metastatic node | |
N1a | 1 metastatic node and micrometastasis1 | |
N1b | 1 metastatic node and macrometastasis2 | |
N2 | 2-3 metastatic nodes | |
N2a | 2-3 metastatic nodes and micrometastasis1 | |
N2b | 2-3 metastatic nodes and macrometastasis2 | |
N2c | 2-3 metastatic nodes and in-transit met(s)/satellite(s) without metastatic nodes | |
N3 | ≥ 4 metastatic nodes, or matted nodes, or in-transit met(s)/satellite(s) with metastatic node(s) | |
(M) Distant Metastasis | ||
M0 | No detectable evidence of distant metastases | |
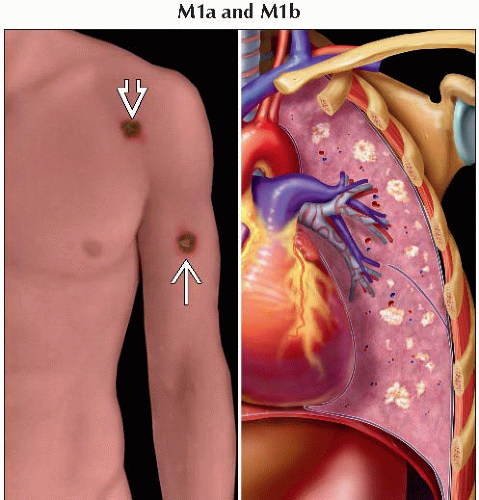
M1a | Metastases to skin, subcutaneous tissues, or distant lymph nodes | |
M1b | Metastases to lung | |
M1c | Metastases to all other visceral sites or distant metastases to any site associated with elevated serum LDH | |
1 Micrometastases are diagnosed after sentinel lymph node biopsy and completion lymphadenectomy (if performed). | ||
Clinical and Pathologic AJCC Stages/Prognostic Groups | Adapted from 7th edition AJCC Staging Forms. | ||||||
Clinical Stage1 | Clinical T | Clinical N | Clinical M | Pathologic Stage2 | Pathologic T | Pathologic N | Pathologic M |
0 | Tis | N0 | M0 | 0 | Tis | N0 | M0 |
IA | T1a | N0 | M0 | IA | T1a | N0 | M0 |
IB | T1b | N0 | M0 | IB | T1b | N0 | M0 |
T2a | N0 | M0 | T2a | N0 | M0 | ||
IIA | T2b | N0 | M0 | IIA | T2b | N0 | M0 |
T3a | N0 | M0 | T3a | N0 | M0 | ||
IIB | T3b | N0 | M0 | IIB | T3b | N0 | M0 |
T4a | N0 | M0 | T4a | N0 | M0 | ||
IIC | T4b | N0 | M0 | IIC | T4b | N0 | M0 |
III | Any T | ≥ N1 | M0 | IIIA | T(1-4)a | N1a | M0 |
T(1-4)a | N2a | M0 | |||||
IIIB | T(1-4)b | N1a | M0 | ||||
T(1-4)b | N2a | M0 | |||||
T(1-4)a | N1b | M0 | |||||
T(1-4)a | N2b | M0 | |||||
T(1-4)a | N2c | M0 | |||||
IIIC | T(1-4)b | N1b | M0 | ||||
T(1-4)b | N2b | M0 | |||||
T(1-4)b | N2c | M0 | |||||
Any T | N3 | M0 | |||||
IV | Any T | Any N | M1 | IV | Any T | Any N | M1 |
1 Clinical staging includes microstaging of the primary melanoma and clinical/radiologic evaluation for metastases. By convention, it should be used after complete excision of the primary melanoma with clinical assessment for regional and distant metastases. | |||||||
Malignant tumor of pigment-producing melanocytes usually located in skin
Relative to other skin malignancies, melanoma is characterized by aggressive nature
Represents small fraction of total skin cancers (4%)
Responsible for majority of deaths from skin cancer (74%)
Incidence of melanoma has been steadily increasing
Melanocytic tumors (WHO classification)
Malignant melanoma
Superficial spreading melanoma
Nodular melanoma
Lentigo maligna
Acral lentiginous melanoma
Desmoplastic melanoma
Melanoma arising from blue nevus
Melanoma arising in giant congenital nevus
Melanoma of childhood
Nevoid melanoma
Persistent melanoma
Major melanoma subtypes (organized by histological characteristics)
Superficial spreading melanoma
Nodular melanoma
Lentigo maligna melanoma
Acral lentiginous melanoma
Desmoplastic melanoma
Comments
Heterogeneous cancer with varying gross pathological and histological characteristics
Location
Can occur anywhere on skin
Risk factors
UV radiation (e.g., sun & tanning beds)
Genetic susceptibilities
Protective factors
Screening skin for concerning lesions
Protection from UV radiation
Sunscreen with zinc oxide &/or titanium dioxide
Number of cases in USA per year
Estimated 2012 statistics for USA
76,250 new cases of melanoma
9,180 deaths from melanoma
Survival rates are improving over time
Sex predilection
1.4:1 male predominance
Age of onset
Wide age range at diagnosis
18% of all patients diagnosed before age 45
Median age of onset is 61 years
Risk factors
Intense or prolonged sun exposure
Increased risk in areas near equator or with sunny climates
Fair-skinned phenotype
History of
Large number of nevi
Dysplastic nevi
Congenital nevi
History of blistering sunburns as child or adolescent
Genetic diseases with highly increased risk
Xeroderma pigmentosum
Familial atypical mole melanoma syndrome
High-risk mutations can lead to uninhibited progression of cell cycle resulting in tumorigenesis
Implicated genes include
CDKN2A (a.k.a. MTS1, p16INK4a, and CDKN2)
CDK4
Moderate- to low-risk mutations include
BRCA2
Retinoblastoma
Melanocortin-1 receptor genes
1p22 gene locus
Hereditary melanomas are linked with increased risk of pancreatic cancer and brain tumors
Characterized by varying morphological appearance
ABCDE criteria
Asymmetry
Border irregularities
Color variegation (varying colors in specified region)
Diameter > 6 mm
Evolution (changes in lesion over time)
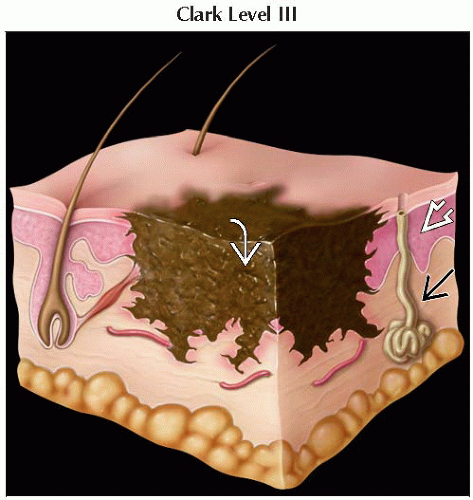
Macular areas correspond to radial growth phase
Raised areas correspond to vertical growth phase
Melanoma exhibits wide degree of histological diversity
Main histologic subtypes
Superficial spreading melanoma
Frequency: ˜ 70% of all melanomas
Location: Trunk in men and women & legs in women
Age: Median onset in 4th-6th decades
Appearance: Commonly exhibits ABCDE warning criteria
Histology
Dominant cells seen in radial and vertical growth phases are epithelioid cells
Epidermis may range from normal to hyperplastic
Diffuse pagetoid pattern is seen
Nodular melanoma
Frequency: ˜ 15-30% of all melanomas
Location: Legs and trunk in men and women
Age: Median onset in 6th decade
Appearance: Often lacks ABCDE warning criteria
May be clinically amelanotic
Commonly involves elevation, ulceration with bleeding, or both
Histology
Lentigo maligna melanoma
Frequency: ˜ 4-15% of all melanomas
Location: Head, neck, and arms in areas of chronically sun-damaged skin
Age: Median onset in 7th decade
Appearance: Development of elevated blue-black nodules within in situ lesion, which often appears dark brown to black
Histology
Often involves a junctional confluent proliferation of melanocytes and extension along adnexal structures
In radial growth phase, lentiginous pattern is seen and atrophy is present in epidermis
Spindle and epithelioid cells are commonly seen in radial and vertical growth phases
Desmoplasia and neurotropism are common
Acral lentiginous melanoma
Frequency: ˜ 2-3% of all melanomas
9-72% of melanomas in dark-skinned individuals (i.e., African-Americans, Asians, & Hispanics)
Only ˜ 2% of melanomas in non-Hispanic whites
Location: Palms, soles, beneath nail plate (subungual)
Age: Median onset in 7th decade
Appearance: Darkly pigmented areas on skin or longitudinal brown, tan, or black streak on a nail bed
Histology
In radial growth phase, lentiginous pattern is seen and epidermis is hyperplastic
Spindle and epithelioid cells are commonly seen in radial and vertical growth phases
Desmoplasia and neurotropism are common
Desmoplastic melanoma
Frequency: < 5% of all melanomas
Location: Sun-exposed areas of head & neck
Age: Median onset in 7th decade
Appearance: Macular area of pigmentation, or firm, amelanotic nodule or scar
Histology: Frequently exhibits deep invasion and perineural involvement
Stains
Immunohistochemistry can offer further diagnostic value in addition to H&E stain
Commonly used monoclonal antibodies that bind to melanoma antigens include
S100
Melan-A/MART-1
HMB-45
Local spread
Some melanomas exhibit in situ growth phase (radial growth) before potential dermal invasion (vertical growth)
e.g., superficial spreading, lentigo maligna, and acral lentiginous melanoma
Nodular melanoma exhibits vertical growth phase from onset
Accounts for its aggressiveness
Desmoplastic melanoma has a propensity for deep invasion with lower risk for nodal and metastatic spread
Lymphatic extension
Most common sites of spread are regional lymph nodes
Autopsy series showed nodal metastases in nearly 3/4 of patients
Satellite metastases: Grossly visible cutaneous &/or subcutaneous metastases occurring ≤ 2 cm of primary
Microsatellites: Microscopic and discontinuous &/or subcutaneous metastases found on pathologic examination adjacent to primary
In transit: Clinically evident cutaneous &/or subcutaneous metastases identified > 2 cm from primary
Hematogenous spread
Lung, brain, distant skin & subcutaneous tissue, liver, nonregional nodes, bone
Primary
Imaging not generally used for detection or assessment of primary because lesion is almost always cutaneous
For rare noncutaneous melanomas, consider contrast-enhanced CT of chest, abdomen, and pelvis, PET/CT, or MR
Nodes
Tc-99m sulfur colloid sentinel lymph node mapping
Directs surgeon to appropriate lymph node basin for sentinel lymph node biopsy
Tracer is injected subcutaneously around site of primary lesion hours before wide local excision
Recommended if Breslow depth > 1 mm or 0.76-1 mm in presence of ulceration &/or ≥ 1 mitoses/mm2
Ultrasound
Abnormal lymph nodes can have abnormal shape, thickened cortex, and loss of fatty hilum
Intraoperative ultrasound can also be performed
CT
Not sensitive for small nodal metastases
Relatively high level of false positives
Metastatic lymph nodes appear round with loss of fatty hilum on CT
Other features suggestive of malignancy include necrosis or abnormal enhancement
PET/CT
In a clinically negative axilla, FDG PET is not generally sensitive for detection of regional lymph node metastases
However, small nodes with increased FDG activity do generally represent metastases
Metastases
CT
Thoracic CECT is main modality in imaging pulmonary metastases
Often > 5 pulmonary metastases seen in metastatic melanoma to lung
Abdominopelvic CT scan
Used in evaluation of metastatic disease to GI tract
Most liver metastases are of lower attenuation than normal parenchyma
MR
Greater sensitivity than CT for CNS metastases
Typical appearance involves multiple areas of T1 hypointensity and heterogeneous T2 hyperintensity
Due to melanin content of tumor, metastases may also appear T1 hyperintense and T2 hypointense
Brain parenchymal lesions show ring, homogeneous, or nodular staging
Lepto-/pachymeningeal enhancement can be seen
Metastases often hemorrhage with characteristic appearance
Hemorrhage also seen in tumors such as renal cell carcinoma, anaplastic lung carcinoma, thyroid carcinoma, choriocarcinoma, and hypernephroma
More accurate in detection of metastases to liver and bone (e.g., spine) than CT
Hepatic metastases ≤ 1 cm have bright signal on T1WI
Detection of bone metastases, especially to spine
PET/CT
Valuable in assessment of possibly resectable stage IV disease
Detection of metastases in advanced disease
More sensitive with either equal or better specificity than CT or MR
Sensitivity greatest in metastases with diameter > 1 cm
≥ 90% sensitivity
PET/CT is possibly superior for bone metastasis, as it has higher sensitivity for osteolytic lesions than Tc-99m MDP bone scanning
Echocardiography
Assesses for cardiac metastases and associated pericardial effusion
Stages I and II
Imaging work-up not required unless there are clinical findings or symptoms suspicious for metastases
Stage IIIA
Consider CT, PET/CT, &/or MR (including brain) for baseline imaging &/or to evaluate specific symptoms
Stages IIIB-C
Recommend CT, PET/CT, &/or MR (including brain) for baseline imaging &/or to evaluate specific symptoms
Stage IV
Encourage CT, PET/CT, &/or MR (including brain) for baseline imaging &/or to evaluate specific symptoms
General
Consider CT, PET/CT, &/or MR to evaluate response to treatment for unresectable or metastatic patients
Elevated laboratory markers or clinical evidence of recurrence should prompt reimaging
e.g., increased serum lactate dehydrogenase (LDH)
PET/CT
FDG PET detects recurrent disease with sensitivity 74% and specificity 86%
Baseline study helpful for assessing response to therapy
Inclusion of upper and lower extremities improves sensitivity
Cytokine therapy promotes symmetric hypermetabolism in normal lymph tissue (tonsils, nodes) for several months
Recent skin biopsy site may show FDG uptake
Non-attenuation-corrected images best for evaluating skin recurrence
Appearance of melanoma upon visual examination of skin surface (ABCDE criteria)
Less common symptoms in pigmented lesion that may indicate melanoma
Bleeding
Itching
Ulceration
Pain
Melanoma often begins as new or changing mole on skin
May grow at cutaneous level before potentially spreading through various routes
Melanoma diagnosed at early stage has relatively better outlook for curative treatment
After metastases, median survival of 6-9 months

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree