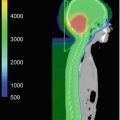
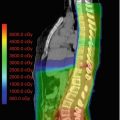
Fig. 6.1
Molecular subgroups of medulloblastoma. Four molecular subgroups have been identified in children. The patient demographic and tumor characteristics are presented (Taylor et al. 2012)
6.5 Workup
Children with medulloblastoma typically will have a magnetic resonance imaging (MRI) scan of the brain prior to resection and diagnosis. Postoperatively, patients undergo a MRI of the brain within 24–48 h after surgery to determine the amount of residual tumor. About 2 weeks after surgery, children with medulloblastoma should have a lumbar tap to rule out malignant cells in the cerebrospinal fluid and a MRI of the entire spine to evaluate for leptomeningeal dissemination. In one study looking at detection of malignant cells, the lumbar tap was more sensitive than ventricular shunt taps (Gajjar et al. 1999). Both lumbar tap and MRI of spine are complementary; if either exam is not done, leptomeningeal dissemination can be missed in about 15% of cases (Fouladi et al. 1999). Bone scan and bone marrow biopsy are only performed nowadays in the rare case of bone pain or abnormal blood count in the setting of leptomeningeal dissemination. Extraneural metastasis is uncommon at initial diagnosis and seen in ≤2% of cases (Mazloom et al. 2010).
The modified Chang staging system is used to note the absence or presence and degree of leptomeningeal spread (Table 6.1) (Chang et al. 1969). In general, about two-thirds of patients will have M0 disease. M1 disease is designated in the presence of malignant cytology from a lumbar tap without MRI evidence of gross tumor spread. Patients with M2 and M3 disease traditionally have worse outcome compared to M1 disease (Harisiadis and Chang 1977).
Table 6.1
Modified Chang staging system for medulloblastoma
Stage | |
|---|---|
M0 | No leptomeningeal spread |
M1 | Malignant cells on cerebrospinal fluid from a lumbar tap |
M2 | Gross leptomeningeal spread in the brain |
M3 | Gross leptomeningeal spread in the spinal axis |
M4 | Extraneural metastasis |
Prior to the age of molecular subtyping of medulloblastoma, the two most important prognostic factors have been the amount of residual in the tumor bed and the M status (Zeltzer et al. 1999). Patients with disease limited to the primary site with less than 1.5 cm2 residual and no dissemination (M0) are classified as having standard- or average-risk disease. Patients with either more than 1.5 cm2 residual or leptomeningeal or extraneural spread (M1–M4) are classified as having high-risk disease.
6.6 Acute Management
Hydrocephalus leading to increased intracranial pressure can be managed by an extraventricular drain, ventriculostomy, or a shunt. In general, most patients with posterior fossa tumors will have an intraoperative extraventricular drain (EVD) if there is significant pre-resection hydrocephalus. The EVD is weaned postoperatively and removed if tolerated. If persistent hydrocephalus remains with inability to wean EVD, a CSF shunt or endoscopic third ventriculostomy is performed (Lin and Riva-Cambrin 2015). The most common type of shunt used is the ventriculoperitoneal shunt. Possible complications of a shunt include malfunction and obstruction, infection, and the rare case of extraneural metastasis.
6.7 Treatment
6.7.1 Surgery
In addition to diversion of CSF to manage hydrocephalus, resection of the primary tumor is an important component of treatment. Maximal safe resection is performed with little or no residual as possible. As stated above, a tumor residual of more than 1.5 cm2 has been associated with a worse survival outcome (Zeltzer et al. 1999). Possible complications of surgery include aseptic meningitis, cervical spine instability, and posterior fossa syndrome. The posterior fossa or cerebellar mutism syndrome is classically seen at 1 or 2 days after surgery and can be accompanied by personality changes, emotional lability, decreased initiation of voluntary movements, and disturbance of ingestion (Pollack 1997). Although it can be associated with surgery for nonneoplastic and neoplastic conditions, children with medulloblastoma are the most commonly affected, and mutism usually lasts for 2–4 months with residual dysarthria. Approximately 8–25% of children with medulloblastoma develop posterior fossa syndrome (Doxey et al. 1999). The underlying cause is still not known, but involvement of the dentate nucleus, bilateral interruption of the dentothalamocortical pathway, and injury to median structures of the cerebellum have been postulated as a possible explanation for this condition. There is some evidence that the incidence of posterior fossa syndrome is increasing, proportional to the increase in more aggressive surgery (Korah et al. 2010).
6.7.2 Radiotherapy
Medulloblastoma is a radiosensitive tumor. The mean inactivation dose and surviving fraction after 2 Gy have been reported to be 1.43 Gy and 27% (Deschavanne and Fertil 1996). RT is a very important component of treatment in the curative management of these patients. Radiotherapy dose, volume, and sequencing of RT and chemotherapy have all been implicated as factors which can influence outcome and are discussed below (Castro-Vita et al. 1980; Garton et al. 1990; Tarbell et al. 1991; Jenkin 1969; Paulino 1997). RT technique is likewise important (Carrie et al. 1992, 1999). It has been previously shown that inadequate coverage of the cribriform plate can lead to a higher risk of subfrontal recurrences (Jereb et al. 1984). Protraction of RT treatment duration for more than 45–50 days has been associated with worse posterior fossa control and survival (Paulino et al. 2003; del Charco et al. 1998; Taylor et al. 2004).
6.7.2.1 Radiotherapy Volume
Historically, the greatest advance in the curative management of medulloblastoma has been the use of craniospinal irradiation (CSI). The landmark Paterson and Farr study established that CSI is the appropriate RT treatment for medulloblastoma (Paterson and Farr 1953). The use of less extensive RT fields (posterior fossa only, posterior fossa and spinal RT) has been associated with worse outcome (Castro-Vita et al. 1980; Jenkin 1969). In the era of chemotherapy, the French M4 protocol utilized chemotherapy to decrease the RT volume to just the posterior fossa and spine (Bouffet et al. 1992). Of 16 children treated, only 3 (18%) were alive and disease-free at a mean follow-up of 6 years. The most common site of relapse was in the supratentorial brain, the site which was not irradiated, accounting for 69% of relapses. Currently, the only children not treated with CSI are the very young patients (<3 years old) where CSI may result in severe neurocognitive and musculoskeletal sequelae (Ashley et al. 2012; Duffner et al. 1993).
After CSI, additional radiation is given to the posterior fossa where the original tumor is located. Historically, the entire posterior fossa (PF) received the boost RT dose. Treatment fields were largely based on using bony landmarks in the skull. Because of advances in imaging and RT treatment delivery and concerns regarding toxicity of RT, some investigators have boosted only the tumor bed and any residual tumor with a safety margin. Current available data indicate that isolated non-tumor bed PF failures occur in <5% of patients when only the tumor bed receives the boost RT dose (Merchant et al. 2008; Paulino et al. 2011; Douglas et al. 2004; Wolden et al. 2003). A study from Toronto suggests that this approach improves neurocognitive outcome compared to treatment of the entire posterior fossa (Moxon-Emre et al. 2014). The recently closed Children’s Oncology Group (COG) ACNS0031 protocol randomized standard-risk patients to PF boost vs. tumor bed boost (tumor bed with a 1.5 cm safety margin for clinical target volume or CTV); the preliminary results of this phase III study showed that the entire posterior fossa does not need to receive the boost dose (Michalski et al. 2016).
6.7.2.2 Radiotherapy Dose
Prior to the era of routine use of chemotherapy, the standard CSI dose was approximately 36 Gy in 20 fractions. In a Children’s Cancer Group study, Packer and colleagues reported a 79% 5-year progression-free survival in standard-risk medulloblastoma with a 23.4 Gy CSI dose followed by a boost with vincristine, lomustine, and cisplatin (Packer et al. 1999). A subsequent COG study randomizing standard-risk patients to two different chemotherapy regimens confirmed the efficacy of 23.4 Gy CSI dose (Packer et al. 2006). Currently, the standard CSI dose for standard-risk patients is 23.4 Gy in 13 fractions followed by a RT boost in conjunction with chemotherapy. When delivering 23.4 Gy CSI for standard-risk patients, RT is started within 30 days of the surgery. The SIOP II study showed an inferior survival outcome for 23.4 Gy CSI patients when RT was delayed, by giving initial chemotherapy; however, one of the criticisms of the study is the possible use of a less efficient chemotherapy regimen (Bailey et al. 1995). The SFOP M7 protocol delivered pre-RT chemotherapy with a reduced supratentorial dose of 27 Gy; RT was delivered at 7 weeks after surgery (Gentet et al. 1995). The 5-year EFS was 74% for patients with macroscopically complete or subtotal excision and M0 disease. The MSFOP 93 nonrandomized protocol delivered pre-RT chemotherapy with RT delivered at the latest 15 weeks after surgery (Oyharcabal-Bourden et al. 2005). RT dose was 25 Gy CSI and 55 Gy PF RT and showed a 5-year recurrence-free survival rate of 64.8%. Although the latter 2 studies suggest that RT can be delayed, the best outcomes that have been reported using a reduced CSI dose with chemotherapy have delivered upfront RT (Packer et al. 1999, 2006).
Some groups have recommended a 36 Gy CSI dose for standard-risk anaplastic medulloblastoma because of their inferior prognosis compared to other histologic subtypes. Currently, the COG ACNS0332 guidelines recommend the use of 36 Gy CSI even for M0 anaplastic medulloblastoma, and this subgroup is currently excluded from the ongoing European PNET5 protocol for standard-risk patients (Chintagumpala et al. 2016).
Studies using a 23.4 Gy CSI dose have shown neurocognitive deficits especially in children younger than 7 years of age (Ris et al. 2001, 2013). Pilot studies from Children’s Hospital of Philadelphia and Indiana University have conflicting results on efficacy and neurocognitive outcome in young children treated with 18 Gy CSI (Goldwein et al. 1996; Jakacki et al. 2004). The COG ACS0031 protocol randomized standard-risk children 3–7 years old to either 18 Gy or 23.4 Gy CSI dose followed by a boost with chemotherapy; the preliminary results inidicate an inferior event-free survival outcome for those treated with 18 Gy CSI (Michalski et al. 2016).
For patients with high-risk disease, CSI doses have typically ranged from 36 to 39.6 Gy in 20 to 22 fractions followed by a posterior fossa/tumor bed boost. Results from the POG 9031 study indicate that the use of this dose regimen with chemotherapy was associated with a favorable 5-year event-free survival (Tarbell et al. 2013). POG 9031 looked at the sequencing of chemotherapy and RT with patients either receiving 3 cycles of cisplatin and etoposide before RT or after RT. Both arms of the study received consolidative vincristine and cyclophosphamide. The 5-year event-free survival (EFS) rates were 66% for chemotherapy first arm and 70% for RT first arm (p = 0.54). The HIT ’91 study showed no difference in EFS or OS among M2 and M3 patients receiving maintenance chemotherapy (RT first) and sandwich chemotherapy (chemotherapy first) (Kortmann 2014; Kortmann et al. 2000). The CSI dose given in this study was 35.2 Gy in 22 fractions with a boost to the posterior fossa for a total dose of 55.2 Gy. In patients with M0 or M1 disease, patients who had RT first had a better EFS and OS. The SIOP/UKCCSG PNET3 tested pre-RT chemotherapy including vincristine, etoposide, carboplatin, and cyclophosphamide followed by 35 Gy CSI and 55 Gy total PF dose for M2–M3 patients with a 5-year PFS of 35% (Taylor et al. 2005).
Some studies have looked at using altered fractionation RT to increase dose for better tumor control. The HIT-SIOP PNET 4 Trial randomized patients with standard-risk medulloblastoma to 23.4 Gy CSI and 54 Gy posterior fossa (1.8 Gy/fraction) over 30 fractions in 6 weeks to 36 Gy CSI, 60 Gy to posterior fossa and 68 Gy to tumor bed (1.0 Gy/fraction given twice daily) over 68 fractions in 6.8 weeks. Both arms included vincristine during RT followed by post-RT 8 cycles of vincristine, lomustine, and cisplatin (Lannering et al. 2012). The 5-year EFS and OS rates were the same in both arms. The PNET 4 showed that hyperfractionation was associated with a better executive function with a trend for better verbal outcomes in the children less than 8 years at the time of RT (Kennedy et al. 2014; Camara-Costa et al. 2015). A study from Mumbai utilizing hyperfractionated RT showed no significant decline in all tested domains of cognitive function in 20 children with a 2-year follow-up (Gupta et al. 2012). Regarding the high-risk group, the Milan strategy for disseminated medulloblastoma included pre-RT chemotherapy, a hyperfractionated accelerated radiotherapy (HART) regimen of 39 Gy CSI (1.3 Gy/fraction twice daily) followed a posterior fossa boost to a total dose of 60 Gy (1.5 Gy/fraction twice daily) and 2 myeloablative courses for persistent disseminated disease before HART (Gandola et al. 2009). The 5-year event-free and overall survival rates were 70 and 73%, comparable to results of the POG 9031 protocol discussed above. To date, the results of altered fractionation RT to increase dose have not shown any significant survival benefit over conventional fractionation.
Table 6.2 outlines some of the major trials performed in standard- and high-risk childhood medulloblastoma (Packer et al. 2006; Bailey et al. 1995; Tarbell et al. 2013; Lannering et al. 2012; von Hoff et al. 2009; Taylor et al. 2003; Gajjar et al. 2006).
Table 6.2
Selected studies in standard- and high-risk medulloblastoma
Study (first author, reference) | Number of patients | Treatment | Outcome |
|---|---|---|---|
SIOP II (Bailey et al. 1995) | 364 SR | Pre-RT chemotherapy vs. upfront RT followed by chemotherapy. Standard-risk randomized to 25 vs. 35 Gy CSI, both followed by boost to total dose of 55Gy | Worse outcome in standard-risk patients receiving pre-RT chemotherapy and 25 Gy CSI compared to patients receiving upfront RT or pre-RT chemotherapy and 35 Gy CSI |
HIT ’91 (von Hoff et al. 2009) | 137 SR HR | Pre-RT chemotherapy vs. upfront RT followed by chemotherapy. CSI dose 35.2 Gy with total dose of 55.2 Gy to PF | Upfront RT better than pre-RT chemotherapy with 10-year OS (91% vs. 62%, p = 0.001) for M0 and (70% vs. 31%) for M1 patients. No difference in OS for M2 and M3 patients |
SJMB-96 (Gajjar et al. 2006) | 134 SR HR | 23.4 Gy CSI for standard and 36–39.6 Gy CSI for high risk followed by boost and four cycles of cyclophosphamide-based dose-intensive chemotherapy | 5-year OS/EFS were 85%/83% and 70%/70% for standard- and high-risk groups |
PNET-3/SIOP III (Taylor et al. 2003) | 179 SR HR (M1 only) | RT alone (35 Gy CSI and 55 Gy total to PF) with or without chemotherapy | Improvement in 5-year EFS (74.2% vs. 59.8%, p = 0.036) but not 5-year OS (76.7% vs. 64.9%, p = n.s.) with chemotherapy |
COG AA9961 (Packer et al. 2006) | 379 SR | 23.4 Gy CSI and 55.8 Gy total dose to PF followed by randomization to 2 chemotherapy regimens (VCR, CDDP, CCNU vs. VCR, CDDP, CPM) | No difference in 5-year PFS (82% vs. 80%) or OS (87% vs. 85%) |
POG 9031 (Tarbell et al. 2013) | 224 HR | Pre-RT chemotherapy vs. Upfront RT. 35.2–40 Gy CSI and 53.2–54 Gy total dose to PF | No difference in 5-year EFS (66% vs. 70%) or OS (73.1% vs. 76.1%) |
MSFOP 98 (Carrie et al. 2009) | 48 SR | Hyperfractionated RT (36 Gy CSI, 60 Gy PF, 68 Gy tumor bed at 1 Gy BID). No chemotherapy | 3-year OS and PFS were 89% and 81% |
HIT-SIOP PNET 4 (Lannering et al. 2012) | 340 SR | Hyperfractionated (36 Gy CSI, 60 Gy PF, 68 Gy tumor bed at 1 Gy BID) vs. conventional RT (23.4 Gy CSI, 54 Gy PF at 1.8 Gy daily) followed by chemotherapy | No difference in 5-year EFS (78% vs. 77%) and OS (85% vs. 87%) |
6.7.3 Chemotherapy
Most patients with medulloblastoma will receive chemotherapy. In infants, chemotherapy is used to delay the institution of RT; in older children, chemotherapy is used to lower the CSI dose in standard-risk patients and improve survival outcome in high-risk patients. Earlier randomized trials have not demonstrated an improvement in survival outcome in patients treated with chemotherapy; however on subset analysis, there is a suggestion that patient with high-risk tumors may have a benefit for the addition of chemotherapy (Krischer et al. 1991; Evans et al. 1990; Tait et al. 1990).
A latter study, SIOP/UKCCSG PNET-3, randomized patients to 35 Gy CSI with 20 Gy posterior fossa boost or chemotherapy followed by the same RT (Taylor et al. 2003). Chemotherapy consisted of vincristine, etoposide, carboplatin, and cyclophosphamide. The 3- and 5-year EFS were 78.5% and 74.2% vs. 64.8% and 59.8% for chemo + RT and RT alone, respectively. There was no statistically significant difference in 3- or 5-year OS.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree