Fig. 17.1
Estrogen and progesterone modulation of fibroid growth
17.3 Medical Treatment of Fibroids
The only treatment for fibroids that has been considered is myomectomy or hysterectomy or, in some cases, limited surgery such as myomectomy by hysteroscopy. Although surgery remains as the unique remedy when bleeding is out of any control, recently, new strategies have been developed to avoid hysterectomy, when possible.
Medical treatment of fibroid is intended to relieve heavy menstrual bleeding to limit anemia and all the related side effects. Unfortunately, at various levels, all these medical options induce anovulation and/or amenorrhea and, in several cases, have contraindications for pregnancy.
Actually, the medical options available are progestins, SERMs, aromatase inhibitors, GnRH analogues, and – the latest innovative remedy – SPRMs.
17.3.1 Progestins
For years, the use of oral or intramuscular progestins has been a classical treatment for abnormal dysfunctional bleeding (Table 17.1) since they act on the endometrium, but no great advantages have been reported on fibroids [6, 15]. This is probably due to the various characteristics of the progestins since some of them induce endometrial atrophy or suppress gonadotropin secretion. However, progestins, as well as P, induce fibroids growth, as above discussed, although some of them show some beneficial effects.
Table 17.1
Actual possible treatments for symptomatic uterine fibroids
Class | Compound | Effects on the patient | Side effects |
|---|---|---|---|
Progestins | NETA NOMAC Danazol LNG Dienogest LNG–IUD | Bleeding control Bleeding control Bleeding control Bleeding control Bleeding control Bleeding control | |
SERMs | Raloxifene | Limited efficacy | Climateric symptoms |
Aromatase inhibitors | Fibroid volume reduction | Climateric symptoms | |
GnRH analogues | Leupreline acetate | Bleeding control, fibroid volume reduction | Climateric symptoms, osteopenia |
SPRMs | Mifepristone Ulipristal acetate Asoprisnil Telapristone acetate | Bleeding control Bleeding control, fibroid volume reduction Bleeding control Bleeding control, fibroid volume reduction | PAECs PAECs PAECs PAECs |
One of the most used progestins is norethisterone acetate (NETA). NETA is a 19-nor-17α-ethynyltestosterone and has been demonstrated to modulate endometrium growth, reducing menstrual bleeding and, to some extent, has been demonstrated to reduce fibroids size with no side effects both when used alone during the luteal phase of the menstrual cycle [16] and when combined with estrogens as hormone replacement therapy.
Nomegestrol acetate (NOMAC), a 19-nor-progesterone derivative, has been demonstrated not to induce fibroid growth when coupled to estrogens both as contraceptive pill and as hormonal replacement therapy [17], but no relevant clinical data have been produced on fibroid treatment. Also danazol and levonorgestrel have been proposed to treat fibroids and with relatively good results [6, 18]. Recently, dienogest, a 19-nor-progesterone derivative, has been demonstrated to reduce leiomyomas growth and size in patients treated for endometriosis similarly to GnRH analogue [19]. Although dienogest has indications for the treatment of endometriosis, it is able to reduce abundant menstrual bleeding induced by fibroids (especially if intramural) since it acts specifically on the endometrium, inducing atresia.
Another interesting option is the intrauterine delivery of progestins by means of an intrauterine device (IUD). The only available system is the one with levonorgestrel, which has been approved by the FDA in the USA to treat abnormal and/or heavy menstrual bleeding [19]. In women with fibroids, it has been demonstrated to reduce menstrual bleeding and to improve anemia, thanks to the induction of atresia of the endometrium, but with minimal effects on the fibroids size [20].
17.3.2 Selective Estrogen Receptor Modulators (SERMs)
This family of drugs is nonsteroidal estrogen ligands and has been shown to have agonist or antagonist effects according to the target tissue. The most known SERM used for breast cancer is Tamoxifen, but for fibroids, Raloxifene has been demonstrated to have an antiestrogenic effect on myomas [21] although the clinical efficacy on fibroids is limited [11].
17.3.3 Aromatase Inhibitors
Aromatase inhibitors belong to a family of steroidal (exemestane) and nonsteroidal (anastrozole, letrozole) drugs that interact with the activity of aromatase. Aromatase is a cytochrome P450 enzyme that transforms androgens into estrogens. Only one trial has been done using letroxole versus GnRH-a, and letroxole reduces fibroids size up to 46 %, but no data were given on bleeding [22].
17.3.4 GnRH Analogue
This is a well-known family of drugs structurally similar to the endogenous GnRH produced by hypothalamus. Their mechanism of action is simple: after binding to the GnRH receptor, there is a stimulation of gonadotropin release (flare-up) and then there is desensitization, blocking any further gonadotropin release. It is obvious that such an action induces within 15 days the dramatic reduction of gonadal steroids plasma levels to the menopausal range. The success of GnRH analogue treatment is due to the induction of amenorrhea to the hypoestrogenic milieu, which block both bleeding and fibroid growth, inducing their shrinking. GnRH analogues have also specific effects on growth factors; decrease paracrine, mitogenic, and angiogenic factors; and induce apoptosis [23, 24]. Treatment with GnRH analogues has been evaluated either alone or with an add-back treatment to reduce the side effects due to the hypoestrogenic condition. Usually, add-back is performed using progestins, estro-progestins, tibolone, and raloxifene, but progestins tend to antagonize GnRH analogue effects on fibroids. Raloxifene further reduces fibroids size but improves the vasomotor symptoms. Tibolone and estro-progestins seem to be less effective in antagonizing GnRH analogue effect and reduce climacteric symptoms significantly.
Although greatly effective on fibroids, GnRH analogues cannot be used for more than 6 months and after suspension fibroid volume increases again [25] together with abnormal bleeding.
17.3.5 Selective Progesterone Receptors Modulators (SPRMs)
SPRMs are a new family of drugs that selectively bind to progesterone receptor, with agonist, antagonist, or mixed activity (Fig. 17.2). Since the beginning ,in vitro experiments showed that fibroid cells treated with SPRMs such as ulipristal acetate (UPA), telapristone acetate, and asoprisnil induced a decrease of cell proliferation and apoptosis, while no effects were observed on normal myometrial cells [26–31].
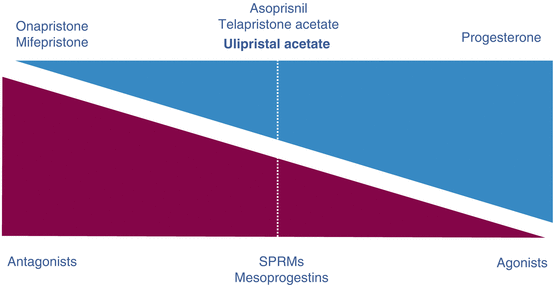

Fig. 17.2
Progesterone receptor ligands show specific activity ranging from pure antagonist to pure agonist (SPRMs: Selective Progesterone Receptor Modulator)
In addition, UPA has been demonstrated to reduce ECM production, which is considered as a relevant determinant for connective and collagen growth [27].
In these last couple of years, a number of clinical studies have investigated the efficacy of SPRMs to treat myomas, with all of them (i.e., mifepristone, UPA, asoprisnil, telapristone acetate) being effective in reducing fibroid size as well as uterus volume [11, 13, 14, 25, 32–35] up to 53 % of their size. In addition, all such SPRMs maintain the reduced fibroid size for longer time interval than do GnRH analogue, up to 6 months after treatment discontinuation [11, 25].
A very interesting aspect is the fact that SPRMs suppress bleeding more rapidly than do GnRH analogues. Indeed, in a randomized trial, bleeding control was achieved and stopped within 7 days after UPA administration [25] compared with 21 days for the GnRH analogue group, and a high percentage of amenorrhea occurred with no hypoestrogenism [25]. Estradiol plasma levels were maintained close to 40–60 pg/ml all along UPA administration, avoiding all climacteric symptoms and all negative side effects on all estradiol-sensitive organs such as bones, bladder, and brain [25].
At present, UPA has been approved as the SPRM for treatment of fibroid at the daily dosage of 5 mg for 3 months [14, 25].
Recently, the treatment interval has been prolonged with UPA at the dosage of 10 mg/day for 3 months for four times with interval of 2 months in between each UPA treatment interval [33]. Such prolongation determined the maintenance of the reduced size of the fibroids together with a high percentage of amenorrheic condition (up to 90 %), and at the end of the last round of treatment, fibroids reached up to −72 % of their starting size [36].
What is interesting to mention is that according to surgeons’ experience, myomectomy that might occur after UPA treatment results to be much easier than those done after GnRH analogue administration since the cleavages of the fibroids are easily found and the amount of bleeding during surgery is much reduced (personal observation).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree