Fig. 42.1
Case no. 1: Stable liver metastases over 4 years demonstrated by high-resolution computerized tomography. Left panel August 2010. Right panel April 2014
Active Surveillance for Progressive Disease
Evaluating serum tumor markers (Ct and CEA) over time in conjunction with obtaining serial cross-sectional imaging is an effective and objective strategy for monitoring for progression of MTC. Serum Ct is the primary biochemical marker used for postoperative surveillance and prediction of disease progression. CEA is a less specific biomarker for MTC but one whose magnitude can be useful in understanding extent of disease and prognosis. Ct and CEA doubling times correlate with rate of progression, recurrence, and survival [1]. Higher disease-specific survival and recurrence-free survival rates are associated with doubling times of over 1 year, with the CEA doubling time having a higher predictive value. Importantly, doubling times can be used to guide the frequency of imaging studies. In patients with prolonged doubling times of over 1 year, the time between imaging studies can be lengthened to every 6–12 months, thus lowering the cost of observation and reducing the radiation exposure risks associated with frequent imaging studies.
A broad spectrum of imaging modalities can be used to detect metastatic disease. Ultrasound of the neck is the most sensitive technique for detection of thyroid bed or nodal recurrence. Areas not easily imaged by ultrasound, such as the retropharyngeal region or superior mediastinum, can be best imaged by computed tomography (CT) or magnetic resonance imaging (MRI) . Fine-section spiral CT or MRI of the chest or abdomen with liver protocol is useful for detection of pulmonary or hepatic metastasis. As case no. 1 clearly demonstrates, spinal metastases occur with some frequency in MTC. In patients with metastatic MTC, it is important to perform periodic bone scans or MRI examination of the spine; however, the presence of a spinal metastasis in the absence of concern about neurologic compressive symptoms is not an indication for therapeutic intervention and may be observed. Although 18F-DOPA PET or a radiolabeled somatostatin analog has utility for detection of MTC, their usefulness for routine surveillance of MTC is unproven.
While RECIST (Response Evaluation Criteria in Solid Tumors) has its limitations, it is a standardized and broadly accepted set of published criteria for evaluating tumor size and determining response and progression in patients with solid tumors, including thyroid cancer (www.recist.org, accessed 10/2014). Progressive disease (PD) is defined as a 20 % increase in target lesion tumor measurements. A 30 % decrease in the size of target lesions defines a partial response (PR); stable disease (SD) is defined as any response in between (−29 to +19 %). A clinical dilemma in real-life practice is that radiologists often do not issue RECIST-based reports, making it incumbent upon the clinician to perform an accurate assessment to determine overall stability or progression.
Integration of Local Therapies into the Management of Progressive or Symptomatic Focal Metastases
Recurrent locoregional neck disease in the setting of distant metastases should be treated surgically if there is impending airway or other vital structural compromise. In selected cases, systemic therapy may be a consideration. For example, in a patient in whom the only surgical option for local airway involvement is total laryngectomy, it may be appropriate to delay surgery and initiate a TKI with the hope that surgery (and loss of voice) can be delayed or the procedure can be modified by reducing the size/extent of the metastatic lesion. Surgical wound healing is impaired by currently available antiangiogenic therapy; thus, such therapy must be stopped between 4 and 12 weeks before a surgical procedure.
It is unclear how targeted systemic therapy should be integrated with the use of external beam radiation therapy (EBRT) . Prior to the availability of TKIs, EBRT was used postsurgically to control extensive nodal or soft tissue neck disease or bone metastases causing pain or neurologic deficit. Indeed, in a patient with localized but incurable disease, palliative EBRT may still be a useful adjuvant therapy, although there is no evidence for overall survival benefit. There is currently a bias (although there are no data supporting it) to defer EBRT to gross disease in the neck in the setting of other progressive, distant disease (and consequently, an indication for systemic therapy), given the risk of upper tracheoesophageal or tracheo-tumor fistula formation in the setting of EBRT to the neck and antiangiogenic therapy [2]. In patients with brain metastases, where there is an inherent risk for bleeding into the brain lesions during treatment with TKIs, which target vascular endothelial growth factor receptor (VEGFR), it is recommended that these lesions be irradiated (EBRT or stereotactic radiosurgery) prior to initiation of TKIs. For this reason, brain imaging should be performed prior to proceeding with systemic therapy. It is important to balance the risks and benefits of EBRT and TKI systemic therapy versus watchful waiting.
Distant metastases limited to the lung that are indolent and asymptomatic can usually be followed with serial imaging. Pulmonary metastases can occasionally cause symptoms such as dyspnea, obstructive pneumonia, and hemoptysis. In selected patients with isolated or localized pulmonary metastases, surgical resection or radiotherapy may offer palliation. For patients with liver metastases that are progressive or symptomatic, trans-arterial chemoembolization or radioembolization can be considered [3].
Skeletal metastases of MTC are often clinically silent. Skeletal related events (SREs) are defined as pain, spinal cord compression, or pathological fracture necessitating external beam irradiation or surgery. It is extremely important to identify bone metastases and initiate palliative treatment modalities before SREs occur. EBRT, stereotactic radiotherapy, vertebroplasty, radiofrequency ablation, cryosurgery, and arterial embolization can be considered to palliate painful metastases or to prevent further progression that could lead to fracture or neurological compromise. Metastasectomy is sometimes performed if there is only one site of bone involvement. Agents that inhibit osteoclast activity, such as bisphosphonates or denosumab, are used in patients with osteolytic bone metastases from several neoplasms (lung, breast, prostate, kidney, and multiple myeloma), and anecdotal experience supports their use in MTC. Zoledronic acid (4 mg) or denosumab (120 mg) given at the approved monthly dosing schedule, in our experience, has been effective in reducing pain and progression of disease associated with bone metastases, although there have been no controlled trials of their use in patients with MTC. Although less frequent dosing of these antiresorptive agents has not been evaluated formally for efficacy in thyroid cancer patients, it is reasonable to surmise that less frequent dosing while maintaining suppression of bone turnover may be as effective in reducing SREs with less risk for side effects, such as osteonecrosis of the jaw. Little is known about the efficacy of TKIs in treating metastatic bone lesions. RECIST excludes bone lesions as measureable targets; thus, bone metastases were not assessed in the phase III trials of vandetanib and cabozantinib although there are some reports of response with these agents [4]. Clinical trials in patients with MTC and bone metastases are needed to evaluate which agents are effective.
Case 2: Treating with a Systemic Agent and Management of Adverse Effects
A 60-year-old man with an indeterminate thyroid mass by fine needle aspiration underwent a total thyroidectomy 12 years ago, which identified sporadic medullary thyroid carcinoma. In that same year, he underwent central lymph node dissection for residual disease. Serum Ct remained detectable around 15 pg/mL (normal is undetectable) for many years. Coincident with rising Ct levels into the 1940s and the identification of recurrent central compartment disease, he underwent a second central lymph node dissection 9 years after original diagnosis. Over the course of the next year, the serum Ct continued to rise (doubling time of 1.5 years), with identification of cervical lateral lymph node metastases and subcentimeter pulmonary (biopsy proven as MTC) and mediastinal lymph node metastases. He was referred to MD Anderson for evaluation. A high-resolution hepatic CT identified suspicious hypervascular hepatic lesions (largest 1.1 cm). At the time, his Ct was 94.6 pg/mL and CEA was 9.4 ng/mL; thus, recommendation was for short-term surveillance for progressive rate. Over the next 7 months, his Ct rose to 250 pg/mL and CEA to 121.8 ng/mL, correlating with asymptomatic, progressive hepatic metastases (largest lesion measured 3 cm). Although systemic chemotherapy with vandetanib or cabozantinib was recommended, the patient elected to be treated with trans-arterial hepatic chemoembolization (TACE) of bilateral hepatic metastases with doxorubicin, as his pulmonary metastases were stable and he was asymptomatic. Reassessment 4 months after TACE identified progressive hepatic and intra-abdominal/cervical lymph node metastases by RECIST associated with rising tumor markers (serum Ct 1144 pg/mL, CEA 452.5 ng/mL). The patient agreed to initiate treatment with one of the approved TKI agents. Three months after TKI initiation, staging studies demonstrated tumoral and biomarker responses (Fig. 42.2).
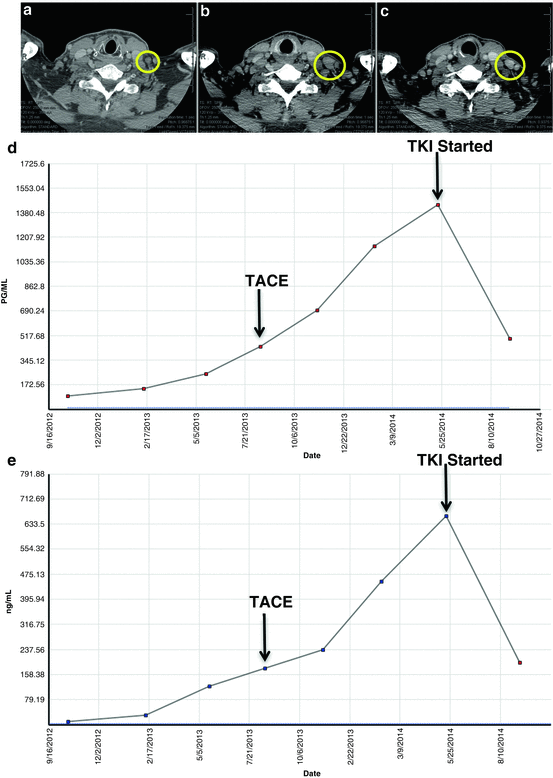

Fig. 42.2




Case no. 2: Cervical lymphadenopathy before and after initiating TKI. (Panel a). Left neck lymph node metastasis, 0.9 cm. (Panel b). Progressive left neck lymph node metastasis, 2.0 cm, 1 year later. TKI initiated. (Panel c) Regression of left neck lymph node metastasis, 1.5 cm, 3 months after initiating TKI. (d) Calcitonin change over time. (e) CEA change over time. TACE trans-arterial chemoembolization, TKI tyrosine kinase inhibitor, CEA carcinoembryonic antigen
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree