When diagnosed at an early stage, resection of pancreatic neuroendocrine tumors (NETs) is often curative. Unfortunately, curative surgery is rarely an option for patients with metastatic disease. Multiple options are available for the management of patients with advanced pancreatic NETs, including surgery, liver-directed therapy, and systemic therapies. Because of the heterogeneity of disease biology and presentation, a multidisciplinary approach to management is critical. Treatment with somatostatin analogs, sunitinib, everolimus, and alkylating agents provide effective systemic therapeutic options for patients. Future studies to evaluate the optimal timing, sequence, and combination of therapies, as well as to identify predictors of response, are warranted.
Key points
- •
Low- and intermediate-grade pancreatic neuroendocrine tumors (NETs) are characterized by variable but most often indolent biological behavior.
- •
Somatostatin analogues decrease hormone production in functional NETs and improve progression-free survival.
- •
The tyrosine kinase inhibitor sunitinib and the mechanistic target of rapamycin inhibitor everolimus improve progression-free survival in patients with progressive pancreatic NETs.
- •
Pancreatic NETs may respond to alkylating agents, including streptozocin and temozolomide.
- •
Studies to evaluate the optimal timing, sequence, and combination of therapies and to identify predictors of response are warranted.
Introduction
Well-differentiated neuroendocrine tumors (NETs) are a rare and heterogeneous group of neoplasms that arise from neuroendocrine cells located throughout the body. These tumors can be broadly classified as either pancreatic NETs or carcinoid tumors, which include NETs arising in other sites, including the thymus, lung, and gastrointestinal (GI) tract. They are characterized by variable but most often indolent biological behavior and are also classically characterized by their ability to secrete peptides resulting in distinctive hormonal syndromes.
Pancreatic NETs compose approximately 1% to 2% of all pancreatic neoplasms. Although NETs have been considered rare, recent studies suggest that they are more common than previously suspected. Analysis of the Surveillance, Epidemiology, and End Results database has demonstrated a significant increase in the incidence of NETs over time, with an age-adjusted annual incidence of pancreatic NETs in the United States of 0.3 cases per 100,000 population. The increase in incidence is likely attributable to increasing awareness, improved diagnostic strategies, and possibly other undetermined environmental and genetic factors.
When pancreatic NETs are diagnosed at an early stage, surgical resection is often curative. Unfortunately, curative surgery is rarely an option for patients with metastatic disease. Until recently, systemic treatment options for patients with advanced pancreatic NETs were limited. However, improvements in our understanding of signaling pathways involved in the pathogenesis, growth, and spread of NETs have translated into an expansion of treatment options. Treatment approaches with somatostatin analogues, agents targeting the vascular endothelial growth factor (VEGF) signaling pathway and the mechanistic target of rapamycin (mTOR), provide therapeutic options for these patients. Cytotoxic chemotherapy may also benefit some patients, particularly those with high disease burden. The aim of this article is to summarize the current and future systemic therapy options for patients with advanced pancreatic NETs.
Introduction
Well-differentiated neuroendocrine tumors (NETs) are a rare and heterogeneous group of neoplasms that arise from neuroendocrine cells located throughout the body. These tumors can be broadly classified as either pancreatic NETs or carcinoid tumors, which include NETs arising in other sites, including the thymus, lung, and gastrointestinal (GI) tract. They are characterized by variable but most often indolent biological behavior and are also classically characterized by their ability to secrete peptides resulting in distinctive hormonal syndromes.
Pancreatic NETs compose approximately 1% to 2% of all pancreatic neoplasms. Although NETs have been considered rare, recent studies suggest that they are more common than previously suspected. Analysis of the Surveillance, Epidemiology, and End Results database has demonstrated a significant increase in the incidence of NETs over time, with an age-adjusted annual incidence of pancreatic NETs in the United States of 0.3 cases per 100,000 population. The increase in incidence is likely attributable to increasing awareness, improved diagnostic strategies, and possibly other undetermined environmental and genetic factors.
When pancreatic NETs are diagnosed at an early stage, surgical resection is often curative. Unfortunately, curative surgery is rarely an option for patients with metastatic disease. Until recently, systemic treatment options for patients with advanced pancreatic NETs were limited. However, improvements in our understanding of signaling pathways involved in the pathogenesis, growth, and spread of NETs have translated into an expansion of treatment options. Treatment approaches with somatostatin analogues, agents targeting the vascular endothelial growth factor (VEGF) signaling pathway and the mechanistic target of rapamycin (mTOR), provide therapeutic options for these patients. Cytotoxic chemotherapy may also benefit some patients, particularly those with high disease burden. The aim of this article is to summarize the current and future systemic therapy options for patients with advanced pancreatic NETs.
Classification of pancreatic neuroendocrine tumors
Several histologic and anatomic classification systems for NETs have been proposed. Although there are differences in the specific criteria for grading tumors, the classification systems reflect the observation that NETs consist of a spectrum of diseases ranging from indolent well-differentiated, low-grade tumors to aggressive poorly differentiated, high-grade tumors ( Table 1 ). In general, tumors with a high histologic grade represent aggressive neuroendocrine carcinomas that have a different natural history and response to treatment compared with low-grade, well-differentiated NETs. In the 2010 World Health Organization (WHO) classification, neuroendocrine neoplasms of the digestive system are categorized as low grade (G1), intermediate grade (G2), and high grade (G3) based on the mitotic count and proliferative (Ki-67) index. High-grade carcinomas have a more aggressive biology and are generally treated with platinum-based chemotherapy regimens used to treat small cell lung cancer. In contrast, well-differentiated, low- and intermediate-grade NETs have lower measures of cell proliferation and a more indolent biology.
| Differentiation | Grade | Mitotic Count a | Ki-67 Index b (%) | Traditional | ENETS, WHO |
|---|---|---|---|---|---|
| Well differentiated | Low grade (G1) | <2 per 10 HPF | ≤2 | Carcinoid, islet cell, pancreatic NET | NET, grade 1 |
| Intermediate grade (G2) | 2–20 per 10 HPF | 3–20 | Carcinoid, atypical carcinoid c , islet cell, pancreatic NET | NET, grade 2 | |
| Poorly differentiated | High grade (G3) | >20 per 10 HPF | >20 | Small cell carcinoma | Neuroendocrine carcinoma, grade 3, small cell |
| Large cell neuroendocrine carcinoma | Neuroendocrine carcinoma, grade 3, large cell |
a Counted in 10 high power fields. High power field = 2 mm 2 at least 40 fields (at 40× magnification) evaluated in areas of highest mitotic density. Cutoffs per AJCC seventh edition.
b MIB1 antibody; percentage of 2000 tumor cells in areas of highest nuclear labeling. Cutoffs per AJCC seventh edition.
c The term atypical carcinoid only applies to intermediate-grade NETs of the lung.
Notably, there is a subset of patients with NETs that seem histologically well differentiated or moderately differentiated but have Ki-67 proliferation indices greater than 20% that fall into the high-grade range. The most appropriate therapy for this heterogeneous subgroup of patients has not been well established. In a retrospective study of 305 patients with G3 neuroendocrine carcinomas of the GI tract (23% with pancreatic primary site), patients with a Ki-67 less than 55% had significantly longer median survival compared with patients with higher Ki-67 indices (14 months vs 10 months). Response rates to platinum-based chemotherapy were lower in patients with a Ki-67 less than 55% (15% vs 42%). Because sensitivity to platinum-based chemotherapy seems to be associated with higher Ki-67 proliferation rates, other cytotoxic agents, such as temozolomide, or targeted agents, such as mTOR inhibitors or VEGF pathway inhibitors, may play a role in the management of well- to moderately differentiated high-grade disease.
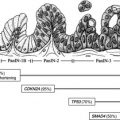
Pancreatic NETs are also classified according to their functional status. Functional tumors, which account for approximately 30% of pancreatic NETs, are associated with clinical syndromes related to hormone secretion. These tumors, including insulinoma, gastrinoma, glucagonoma, and vasoactive intestinal peptide (VIPoma), are named according to the hormone that is secreted. In contrast, nonfunctional tumors include those that are not associated with a specific clinical syndrome related to hormone secretion.
Genetic basis of neuroendocrine tumors
Inherited Neuroendocrine Tumor Syndromes
Most pancreatic NETs occur as nonfamilial (sporadic) tumors. However, several autosomal dominant genetic syndromes, including multiple endocrine neoplasia type 1 (MEN1), von Hippel-Lindau syndrome (VHL), neurofibromatosis type 1 (NF-1), and tuberous sclerosis (TS), have been associated with the development of NETs. MEN1 is caused by inactivating mutations of the MEN1 gene. VHL results from germline mutations in the VHL gene, which functions as a tumor suppressor gene that regulates hypoxia-induced cell proliferation and angiogenesis. NF-1 and TS are caused by inactivating mutations in the tumor suppressor genes NF1 and TSC1 and TSC2 , respectively. NF1 encodes the protein neurofibromin, which regulates TSC1 and TSC2 . TSC1 and TSC2 form a tumor suppressor heterodimer that inhibits mTOR . Although most NETs are sporadic, the molecular genetics of these tumor susceptibility syndromes provide insight into the genetic mechanisms of this disease.
Recent efforts have also focused on the genetic basis of sporadic, nonfamilial pancreatic NETs. In a study involving exome sequencing of nonfamilial pancreatic NETs, Jiao and colleagues found that the most frequently mutated genes encoded proteins involved in chromatin remodeling. Forty-four percent of tumors had somatic inactivating mutations in MEN1 , and 43% had mutations in genes encoding either death-domain-associated protein ( DAXX ) or α thalassemia/mental retardation syndrome X-linked ( ATRX ). Mutations in genes in the mTOR pathway occurred in 14% of tumors. Furthermore, mutations in MEN1 and DAXX/ATRX genes may identify a biologically distinct subgroup of pancreatic NETs with a favorable prognosis. Mutations in MEN1, DAXX/ATRX , or the combination of these genes were associated with improved survival; 100% of patients with mutations in both MEN1 and DAXX/ATRX survived at least 10 years in contrast to death within 5 years of diagnosis for 60% of patients without these mutations. Studies are ongoing to investigate whether the mutational profile is a predictive response to chemotherapy or targeted agents, including mTOR inhibitors.
Systemic treatment of advanced pancreatic neuroendocrine tumors
Multiple options are available for the management of patients with advanced, metastatic pancreatic NETs, including surgical resection, liver-directed therapies, and systemic therapy. Because of the heterogeneity of disease biology and presentation, a multidisciplinary approach to management is critical. The goals of therapy are to improve symptoms related to hormone hypersecretion, slow disease progression, and improve survival. Systemic therapy options include somatostatin analogue therapy, cytotoxic chemotherapy, and targeted agents, including everolimus and sunitinib.
Somatostatin Analogues
Somatostatin is a natural 14-amino acid peptide that binds to G-protein-coupled somatostatin receptors (SSTRs) that are expressed on most NETs. Of the 5 different SSTR subtypes, SSTR-2 is expressed in approximately 80% of pancreatic NETs, with the exception of insulinomas, which express SSTR-2 in less the 50% of cases. By binding to somatostatin receptors, somatostatin analogues, including octreotide and lanreotide, have both antisecretory and antiproliferative effects.
Somatostatin analogues and control of symptoms from hormone secretion
Patients with metastases from functional pancreatic NETs often become symptomatic from hormone hypersecretion rather than from tumor bulk. Symptoms related to hormone secretion can often be well controlled with somatostatin analogues. The role of somatostatin analogues has been best established for patients with VIPoma and glucagonoma. Overproduction of vasoactive intestinal peptide can result in severe secretory diarrhea and hypokalemia. These tumors are very responsive to administration of somatostatin analogues, which can reduce VIP levels and improve symptoms. In patients with glucagonoma, reduction in glucagon levels and improvement in the characteristic rash (necrolytic migratory erythema) are observed in most patients with the use of somatostatin analogues.
Although insulinomas and gastrinomas represent the most common types of functioning pancreatic NETs, the role of somatostatin analogues in controlling hormone-related symptoms for these tumor types is less well established. In patients with gastrinoma, high-dose proton pump inhibitors can effectively control hypergastrinemia-related gastric acid production and remain a mainstay of treatment in these patients. In patients with insulinoma, only 50% of patients express SSTR-2. In patients without SSTR-2 expression, hypoglycemia may paradoxically worsen because of inhibition of glucagon secretion caused by somatostatin analogue therapy. Therefore, patients with insulinoma need to be closely monitored when initiating therapy with somatostatin analogue therapy.
Somatostatin analogues and disease control
The antiproliferative effects of somatostatin analogues occur through both direct and indirect mechanisms. Binding of somatostatin analogues to SSTR-2 and SSTR-5 can lead to arrest of mitosis and cell cycle and may also induce apoptosis. The indirect antiproliferative effects of somatostatin analogues may be mediated through decreased production of circulating growth factors and inhibition of angiogenesis via reduction in production and release of proangiogenic factors.
Although objective tumor shrinkage with somatostatin analogues is rare, tumor growth may be slowed. In the PROMID study, 85 patients with inoperable or metastatic well-differentiated midgut NETs were randomized to receive octreotide LAR 30 mg monthly or placebo. Median time to tumor progression was significantly longer for patients receiving octreotide (14.3 vs 6.0 months). A limitation of this study, however, was that it did not include patients with pancreatic NETs.
More recently, however, support for the antiproliferative effect of somatostatin analogues in pancreatic NETs was provided by the phase III CLARINET trial, which compared lanreotide versus placebo in 204 patients with advanced well- or moderately differentiated, nonfunctioning GI and pancreatic (45%) NETs. Patients were randomly assigned to receive either 120 mg lanreotide Autogel or placebo every 4 weeks for 96 weeks or until progressive disease or death. All patients had avid disease on SSTR scintigraphy. Most patients (96%) had no tumor progression in the 3 to 6 months before randomization. Compared with placebo, lanreotide was associated with significantly prolonged progression-free survival (PFS), with a similar effect seen across major subgroups. At a time point of 2 years following initiation of treatment, the median PFS was not reached with lanreotide compared with 18 months with placebo (hazard ratio [HR] for progression or death 0.45; 95% confidence interval [CI] 0.30–0.73). Based on these data, lanreotide has been approved in the United States for the treatment of patients with unresectable, well- or moderately differentiated, locally advanced or metastatic gastroenteropancreatic NETs.
Targeted therapy
Mechanistic Target of Rapamycin Inhibitors
The mTOR (also referred to as mammalian target of rapamycin) is an intracellular serine/threonine kinase that regulates key cell functions involved in cell survival, proliferation, and metabolism. Signaling through the PI3K (phosphatidylinositide 3-kinase)/AKT/mTOR pathway leads to increased translation of proteins regulating cell cycle progression and metabolism. mTOR mediates downstream signaling from several pathways, including VEGF and insulin-like growth factor (IGF), that are implicated in NET growth. Several observations support the importance of the mTOR pathway in the pathogenesis of NET. First, although most NETs arise sporadically, NETs can arise within the context of several familial cancer syndromes, including NF-1 and TSC, that are due to inactivating mutation in tumor suppressor genes leading to activation of the mTOR pathway. Additionally, gene expression analyses have demonstrated altered expression of genes in the mTOR pathway, and recent gene sequencing studies of pancreatic NETs have revealed mutations in genes in the mTOR pathway in 14% of tumors.
Everolimus monotherapy was compared with best supportive care alone in the placebo-controlled RADIANT-3 trial, which included 410 patients with advanced pancreatic NETs ( Table 2 ). Approximately 40% of patients also received somatostatin analogue therapy. Everolimus was associated with a significant prolongation in median PFS (11.0 vs 4.6 months, HR for progression 0.35, 95% CI 0.27–0.45). Confirmed objective partial radiographic responses were observed in 5% of patients receiving everolimus compared with 2% of those receiving placebo. The rate of tumor stabilization was high, 73% among patients receiving everolimus versus 51% in the placebo group. Based on this result, everolimus was approved by the Food and Drug Administration (FDA) for patients with progressive pancreatic NETs. Drug-related adverse events included stomatitis, rash, diarrhea, and fatigue. The most common grade 3 or 4 drug-related adverse events were stomatitis (7%), anemia (6%), and hyperglycemia (5%). Although rare, everolimus has been associated with serious adverse events, including pneumonitis.
| Study | Agent | No. Patients | Tumor Response Rate (%) | Median TTP ( T ) or PFS ( P ) | Reference |
|---|---|---|---|---|---|
| Phase II studies | |||||
| RADIANT-1 | Everolimus | 115 | 9 | 9.7 mo P | Yao et al, 2010 |
| Everolimus + octreotide | 45 | 4 | 16.7 mo P | ||
| Temsirolimus a | 15 | 7 | 10.6 mo T | Duran et al, 2006 | |
| Temsirolimus + bevacizumab | 58 | 41 | 13.2 mo P | Hobday et al, 2014 | |
| Phase III studies | |||||
| RADIANT-3 | Everolimus | 207 | 5 | 11.0 mo P | Yao et al, 2011 |
| Placebo | 203 | 2 | 4.6 mo P | ||
| CALGB 80701 | Everolimus | 75 | 12 | 14.0 mo P | Kulke et al, 2015 |
| Everolimus + bevacizumab | 75 | 31 | 16.7 mo P | ||
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree