Chapter 6 Mechanisms of Innate Immunity
• Innate immune responses do not depend on immune recognition by lymphocytes, but have co-evolved with and are functionally integrated with the adaptive elements of the immune system.
• The body’s responses to damage include inflammation, phagocytosis, and clearance of debris and pathogens, and remodeling and regeneration of tissues. Inflammation is a response that brings leukocytes and plasma molecules to sites of infection or tissue damage.
• The phased arrival of leukocytes in inflammation depends on chemokines and adhesion molecules expressed on the endothelium. Adhesion molecules fall into families that are structurally related. They include the cell adhesion molecules (CAMs) of the immunoglobulin supergene family (which interact with leukocyte integrins), and the selectins (which interact with carbohydrate ligands).
• Leukocyte migration to lymphoid tissues is also controlled by chemokines. Chemokines are a large group of signaling molecules that initiate chemotaxis and/or cellular activation. Most chemokines act on more than one receptor, and most receptors respond to more than one chemokine.
• Plasma enzyme systems modulate inflammation and tissue remodeling. The kinin system and mediators from mast cells including histamine contribute to the enhanced blood supply and increased vascular permeability at sites of inflammation.
• Pathogen-associated molecular patterns (PAMPs) or microbial-associated molecular patterns (MAMPs) are distinctive biological macromolecules that can be recognized by the innate immune system. Innate antimicrobial defenses include molecules of the collectin, ficolin, and pentraxin families, which can act as opsonins, either directly or by activating the complement system. Macrophages have cell-surface scavenger-receptors and lectin-like receptors, which allow them to directly bind to pathogens and cell debris.
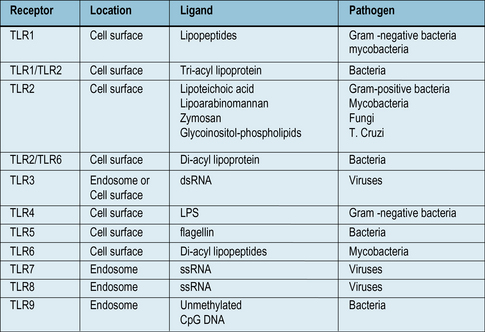
• Toll like receptors (TLRs) are a family of receptors that recognize PAMPs from bacteria, viruses and fungi. They are present on many cell types, and can activate macrophages, using signaling systems that are closely related to those used by inflammatory cytokines TNFα and IL-1.
• Intracytoplasmic pattern recognition receptors (PRRS) recognize products of intracellular pathogens. Receptors of the Nod family recognize bacterial products, while the RLH receptors can recognize products of viral replication.
Innate immune responses
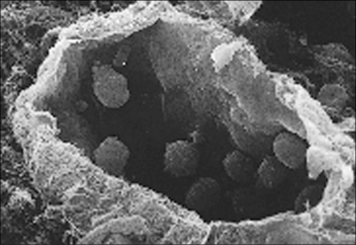
The adaptive immune responses depend on the recognition of antigen by lymphocytes, a cell type that has evolved relatively recently – lymphocytes are present in all vertebrates, but not invertebrates, although lymphocyte-like cells are present in closely related phyla, including the tunicates and echinoderms (Fig. 6.1).
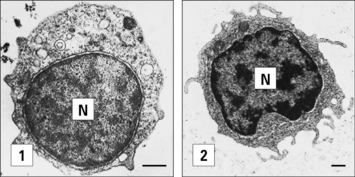
Fig. 6.1 Electron micrographs of lymphocyte-like cells
(Courtesy of Dr AF Rowley from Endeavour 1989:13;72–77. Copyright 1989 with permission from Elsevier.)
• displays the very primitive immune defense of phagocytosis; but also
• expresses MHC molecules and acts as an antigen-presenting cell, a function that makes sense only in relation to the evolution of T cells.
We can identify some of the ancient innate immune defense systems because related systems are seen in distant phyla. For example, the family of Toll-like receptors (TLRs, see Fig. 6.21) were first identified in insects. We can therefore infer that the distant ancestor of mammals and insects had a receptor molecule of this type that probably recognized microbial components.
Inflammation – a response to tissue damage
The body’s response to tissue damage depends on:
• killing of pathogens, neutralizing toxins, limiting pathogen spread;
• phagocytosis of debris, pathogens, and dead cells;
• proliferation and mobilization of fibroblasts or other tissue cells to contain an infection and/or repair damage;
• removal or dissolution of blood clots and remodeling of the components of the extracellular matrix;
• regeneration of cells of the tissue and re-establishing normal structure and function.
Inflammation brings leukocytes to sites of infection or tissue damage
Q. What three principal changes occur in the tissue during an acute inflammatory response?
A. An increased blood supply to the affected area, an increase in capillary permeability allowing larger serum molecules to enter the tissue and an increase in leukocyte migration into the tissue (see Chapter 1).
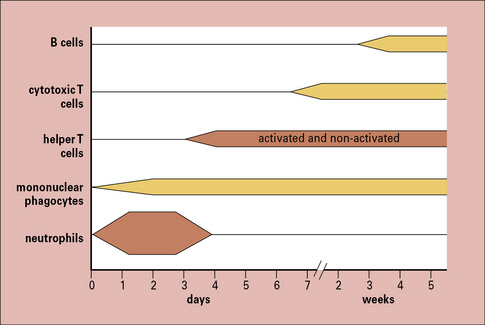
The cells seen in acute and chronic inflammation are quite different, and reflect the phased arrival of different populations of leukocytes into a site of infection (Fig. 6.2). Consequently:
• sites of acute inflammation tend to have higher numbers of neutrophils and activated helper T cells; whereas
• sites of chronic inflammation have a higher proportion of macrophages, cytotoxic T cells, and even B cells.
Cytokines control the movement of leukocytes into tissues
Tissue damage leads to the release of a number of inflammatory cytokines, either from:
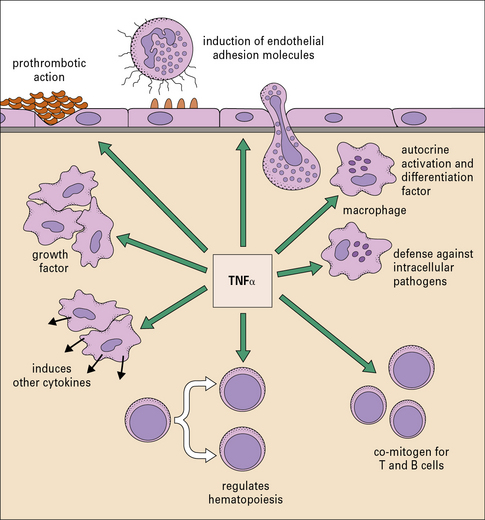
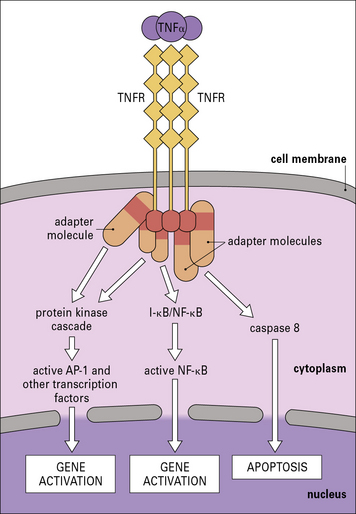
The cytokines tumor necrosis factor-α (TNFα), IL-1 and interferon-γ (IFNγ) are particularly important in this respect. TNFα is produced primarily by macrophages and other mononuclear phagocytes and has many functions in the development of inflammation and the activation of other leukocytes (Fig. 6.3). Notably, TNFα induces the adhesion molecules and chemokines on the endothelium, which are required for the accumulation of leukocytes. TNFα and the related cytokines, the lymphotoxins, act on a family of receptors causing the activation of the transcription factor NF-κB (Fig. 6.4), which has been described as a master-switch of the immune system. NF-κB is, in fact, a group of related transcription factors, which can also be activated by Toll-like receptors and IL-1. The activation of vascular endothelium by TNFα or IL-1 causes chemokine production and adhesion molecules to be expressed on the endothelial surface.
Leukocytes migrate across the endothelium of microvessels
The routes that leukocytes take as they move around the body are determined by interactions between:
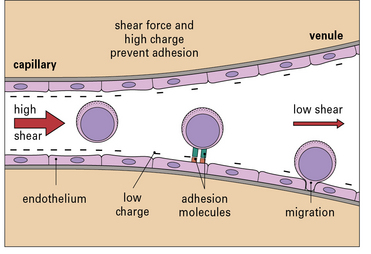
Leukocyte migration is controlled by signaling molecules, which are expressed on the surface of the endothelium, and occurs principally in venules (Fig. 6.5). There are three reasons for this:
• the signaling molecules and adhesion molecules that control migration are selectively expressed in venules;
• the hemodynamic shear force in the venules is relatively low, and this allows time for leukocytes to receive signals from the endothelium and allows adhesion molecules on the two cell types to interact effectively;
• the endothelial surface charge is lower in venules (Fig. 6.6).
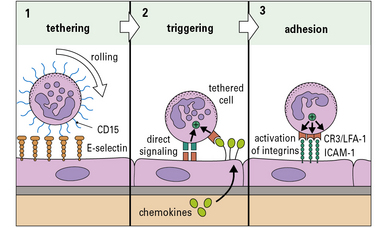
Although the patterns of leukocyte migration are complex, the basic mechanism appears to be universal. The initial interactions are set out in a three-step model (Fig. 6.7):
• Step-1 leukocytes are slowed as they pass through a venule and roll on the surface of the endothelium before being halted – this is mediated primarily by adhesion molecules called selectins interacting with carbohydrates on glycoproteins;
• Step-2 the slowed leukocytes now have the opportunity to respond to signaling molecules held at the endothelial surface – particularly important is the large group of cytokines called chemokines, which activate particular populations of leukocytes expressing the appropriate chemokine receptors;
• Step-3 activation upregulates the affinity of the leukocytes’ integrins, which now engage the cellular adhesion molecules on the endothelium to cause firm adhesion and initiate a program of migration.
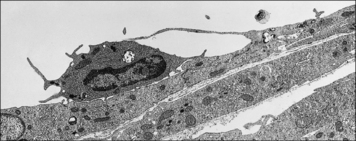
Transendothelial migration is an active process involving both leukocytes and endothelial cells (Fig. 6.8). Generally leukocytes migrate through the junctions between cells, but in specialized tissues such as the brain and thymus, where the endothelium is connected by continuous tight junctions, lymphocytes migrate across the endothelium in vacuoles, near the intercellular junctions, which do not break apart.
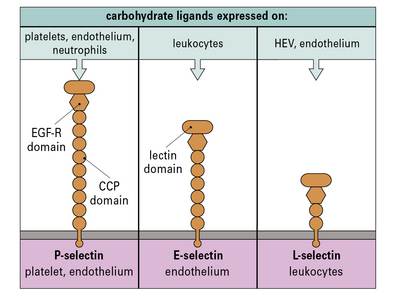
Selectins bind to carbohydrates to slow the circulating leukocytes
• E-selectin and P-selectin, which are expressed predominantly on endothelium and platelets; and
• L-selectin, which is expressed on some leukocytes (Fig. 6.9).
The carbohydrate ligands for the selectins may be associated with several different proteins:
• at sites of inflammation, E-selectin and P-selectin, which are induced on activated endothelium, bind to the sialyl Lewis-X carbohydrate associated with CD15, present on many leukocytes;
• some of the selectin ligands are selectively expressed on particular populations of leukocytes, for example the molecule PSGL-1 (P-selectin glycoprotein ligand) present on TH1 cells binds to E- and P-selectin, but a variant found on TH2 cells does not.