Fig. 6.1
Hypothalamic-pituitary-gonadal (HPG) axis and puberty according to [49]. Hypothalamic GnRH neurons receive trans-synaptic inhibitory and excitatory inputs that modulate the pulsatile release of GnRH. This neuropeptide drives the secretion of FSH and LH from the anterior pituitary, which in turn stimulates the secretion of gonadal sex steroids. These steroids have both negative and positive feedback effects for estradiol (E2) or only negative one for testosterone (T). Leptin produced by the white adipose tissue (WAT) is involved in the metabolic regulation of HPG
6.2 Neuronal Control of Puberty: Essential Roles of GnRH and Kisspeptin (KP) Neurons
During puberty onset, GnRH pulsatility increases in both amplitude and frequency and stimulates the gonadotroph cells of the anterior pituitary gland. This increases the secretion of FSH and LH, which in turn stimulates the secretion of sex steroids: testosterone by the Leydig cells of the testis and androstenedione by the thecal cells of the ovarian follicles, which is then converted into estrogen by the aromatase enzyme in granulosa cells [4]. The increase in sex steroids stimulates the somatotroph axis, causing growth acceleration, progressive growth plate maturation, and the development of secondary sexual characteristics, all of which lead to the clinical expression of pubertal development. This key role of GnRH neurons has been clearly demonstrated by the clinical model of Kallmann’s hypogonadotropic hypogonadism due to a migration defect of GnRH neurons [43]. Nevertheless, pubertal changes in pulsatile GnRH secretion are induced by modifications in the axo-dentritic inputs to the GnRH neuronal network [24, 35]. Puberty may thus be the end point in the hierarchical activation/inactivation of excitatory and inhibitory GnRH regulators [35].
Among the neuropeptide regulators of GnRH neurons, kisspeptin (KP) has been recognized as essential for the control of puberty onset [48]. KP is encoded by the Kiss1 gene and operates through the kisspeptin receptor (KissR), a G protein-coupled receptor also called Gpr54 . In rodents, two prominent populations of KP neurons have been reported in the arcuate nucleus (ARC) and the rostral periventricular area of the third ventricle (RP3V) of the hypothalamus, whereas in other mammals including primates, KP neurons are significantly expressed in the ARC/infundibular region [38]. In recent years, genetic endocrine disorders have proved useful for gaining a better understanding of physiological processes. With regard to puberty, inactive mutations of the Kiss1 gene or Gpr54 gene result in hypogonadotropic hypogonadism [12, 44]. Conversely, activating mutations of either of these genes lead to precocious puberty [46, 47].
Experimental data have also pointed to the key role of KP neurons in puberty onset. First, a rise in hypothalamic expression of the Kiss1 and Gpr54 genes has been reported during pubertal transition in rats [30]. Second, KP in mice elicits a better LH response in adults than in prepubertal mice, giving evidence of an increase in the sensitivity to the stimulatory effects of KP on GnRH/LH secretion [21]. The increase in the depolarizing KP action on GnRH neurons from juvenile to adult age reinforces the better Gpr54 signaling efficiency during the pubertal period [21]. Last, an elevation in the number of KP-positive neurons and their projections toward GnRH neurons has been also demonstrated at the time of puberty [11]. This mechanism is more marked in female than in male mice, leading to the hypothesis of a sexual dimorphism in Kiss1 gene expression.
6.3 Roles of KP Partners in Pubertal Onset
In addition to the crucial role of KP in the control of puberty onset, other endocrine signals have been reported to modulate GnRH neuron activity by several groups.
Anatomical analyses have demonstrated that a high percentage of KP neurons co-expresses neurokinin B (NKB) and its receptor (NK3R) in the ARC [20, 32, 49]. Since most of these neurons also express dynorphin-A (Dyn), they have been termed KNDy neurons [25]. As for KP, pubertal disorders underline the role of NKB and its receptor in pubertal regulation. For example, Topoglu reported four human pedigrees with severe congenital gonadotrophin deficiency and pubertal failure that were homozygous for loss-of-function mutation in TAC3 (encoding NKB) or TACR3 (encoding NK3R) [50]. These data, as well as the demonstration of an increase in TAC3 and TACR3 expression during puberty in mice [19], suggest that NKB is a central regulator of human gonadotropic function. Moreover, numerous experimental studies have reported a potent stimulatory action of the
NKB agonist senktide on LH secretion [18]. This stimulatory effect is nevertheless absent in Gpr54 null mice, suggesting that KP signaling may be a link between NKB and GnRH neurons [18]. In support of this hypothesis, experimental data demonstrate the stimulatory action of the NKB agonist senktide on KP neurons, but not directly on GnRH neurons [31]. In parallel, it was demonstrated that KNDy neurons express TACR3 gene, whereas GnRH do not [31]. All these data confirm that NKB activates GnRH neurons indirectly through kisspeptin [32].
Sex steroid levels are also involved in the regulation of pubertal onset. It is well known that sex steroids have both negative and positive feedback effects [4]. This has been corroborated by several clinical reports of central precocious puberty occurring during peripheral pubertal progression [37]. Recent works have underlined that the ARC hypothalamic area may be involved in the negative feedback of estrogens (E), whereas the rostral periventricular region of the hypothalamus (AVPV) is involved in its positive feedback [24]. Comparisons of wild type and ERα knock-out mice revealed that both negative and positive estrogen feedback are mediated by ERα [10]. Nevertheless, ERβ, but not ERα, is expressed in GnRH neurons, raising a question about the link between E and GnRH neurons [23]. A good candidate for this interaction would again be the KNDy neurons. First, increased Kiss1 gene expression was reported in postmenopausal women and ovariectomized monkeys [41]. Conversely, estrogen replacement in the monkeys markedly reduced Kiss-1 gene expression [41]. Second, the ablation of KNDy neurons prevents the rise of LH in ovariectomized mice [29]. Nevertheless, the positive feedback of E2 on the preovulatory LH surge is not fully understood, although it may involve estrogen-receptive histamine-containing neurons [16, 24].
6.4 Metabolic Control of Puberty
A substantial quantity of data points to the essential role of metabolic factors in the control of puberty. First, the concept of a critical fat mass – that is, the necessity of a minimal weight for normal puberty – emphasizes the role of leptin produced by adipose tissue [8]. In addition, leptin and leptin receptor deficiency in humans are known to be responsible for hypogonadotropic hypogonadism [14, 15], while overweight and obese prepubertal girls present earlier puberty onset [2]. These data underline the important role of leptin in puberty. However, the normalization of mean leptin concentrations in the condition of negative energy balance failed to restore normal gonadotropin levels in female rats [51]. Moreover, pharmacological studies in humans and rodents with leptin deficiency have clearly demonstrated that leptin alone cannot trigger early puberty [9]. All these data suggest that rather than being a trigger, leptin plays a permissive role in puberty onset [40]. Other impacts of leptin at other levels of the hypothalamic-pituitary-gonadal (HPG) axis have also been considered. This principal central leptin action seems indirectly mediated since leptin receptor (LepR) mRNA was not found in GnRH neurons [39, 42]. It is currently unknown where leptin conducts its reproductive actions. Negative energy balance was reported to induce suppression of Kiss1 mRNA in pubertal rat hypothalamus, whereas administration of KP increased both serum LH levels and hypothalamic Kiss1 mRNA levels [6]. On the other hand, premature onset overweight in female rats increased Kiss1 gene expression [7]. Although these data stress the important role of leptin in GnRH pubertal activation via the KP neurons, one issue remains unresolved: does leptin have a direct or indirect action on the KP neurons? The above-cited works have demonstrated that leptin exerts both a direct and an indirect action on KP neurons. The detection of LepR mRNA in KP neurons of the ARC strengthens the assumption of direct leptin action on these neurons [5]. Nevertheless, not only congenital selective ablation of these LepR from KP neurons is compatible with preserved puberty and fertility [13], but LepR is also expressed in various hypothalamic area [28] underlying an indirect action of leptin via pathways that have yet to be identified.
6.5 Novel Mechanism in the Control of Puberty
As described above, substantial efforts have been made in the past decade to identify the factors involved in the initiation of puberty. This body of work has not only shown the major role of the KP/GPR54 system in pubertal development, but has also indicated that the initiation of puberty requires the involvement of many other genes such as TAC, TACR3, Lep, and LepR. A new concept of pubertal initiation is related to upstream mechanisms involving gene silencing (Fig. 6.2). These upstream mechanisms can also include epigenetic regulation [33]. Kiss1 gene was thus found to be repressed in the ARC before puberty by the polycomb group transcriptional silencing complex (EED and CBX7) [26]. DNA methylation of two key members of this complex lifted this repression at the initiation of puberty, which coincided with an increase in the expression of the Kiss1 gene. Further insight into pubertal regulation was recently provided by several cases of familial central precocious puberty due to MKRN3 mutations [1, 45]. MKRN3 is a maternally expressed gene that encodes the protein MKRN3, whose transcription decreases in mice during peripuberty. These humans as well as experimental data strongly suggest that MKRN3 represses the initiation of puberty during the juvenile pause [1].
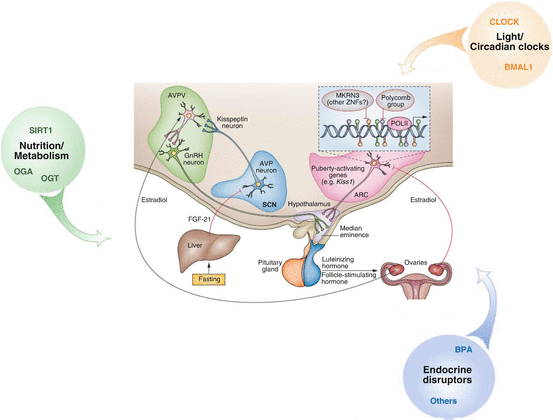

Fig. 6.2
The repressive control of puberty according to [27, 33]. The polycomb group complex and the MKRN3 are the two gene-silencing systems expressed in the ARC. The loss of transcriptional repression identified as a main mechanism underlying the onset of puberty. Different external and internal environmental stimuli (nutrition/metabolism, light/circadian clocks, endocrine disruptors) also modulate the timing and progression of puberty. Abbreviation: SCN suprachiasmatic nucleus, ARC arcuate nucleus, AVPV anteroventral periventricular nucleus, AVP vasopressin, MKRN3 markorin
Conclusion
Puberty is not a punctual phenomenon. It is the culmination of a general maturation process that starts in fetal life, is reactivated in the neonatal period, and is finally expressed in adolescence. Pubertal timing is shaped by the dynamic interplay between gene load and the environment. Puberty has thus been regarded as a biological sensor, and downward trends in the timing of puberty, as have been detected in recent years in wildlife species and human populations, are considered as potential biomarkers for the suspected deterioration of the hypothalamic-pituitary-gonadal axis due to environmental influences. Our understanding of pubertal regulation has progressed greatly over the past decade, especially with the identification of novel neuropeptide pathways (Kiss, NKB, etc.) and molecular mechanisms (epigenetics, mRNA) involved in its regulation.
References
1.
Abreu AP, Dauber A, Macedo DB, Noel SD, Brito VN, Gill JC, Cukier P, Thompson IR, Navarro VM, Gagliardi PC, Rodrigues T, Kochi C, Longui CA, Beckers D, de Zegher F, Montenegro LR, Mendonca BB, Carroll RS, Hirschhorn JN, Latronico AC, Kaiser UB (2013) Central precocious puberty caused by mutations in the imprinted gene MKRN3. N Engl J Med 368:2467–2475CrossRefPubMed
2.
3.
Aksglaede L, Sorensen K, Petersen JH, Skakkebaek NE, Juul A (2009) Recent decline in age at breast development: the Copenhagen Puberty Study. Pediatrics 123:e932–e939CrossRefPubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





