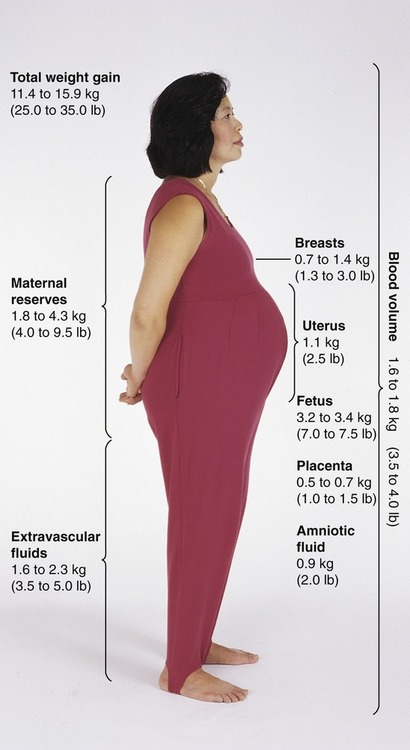
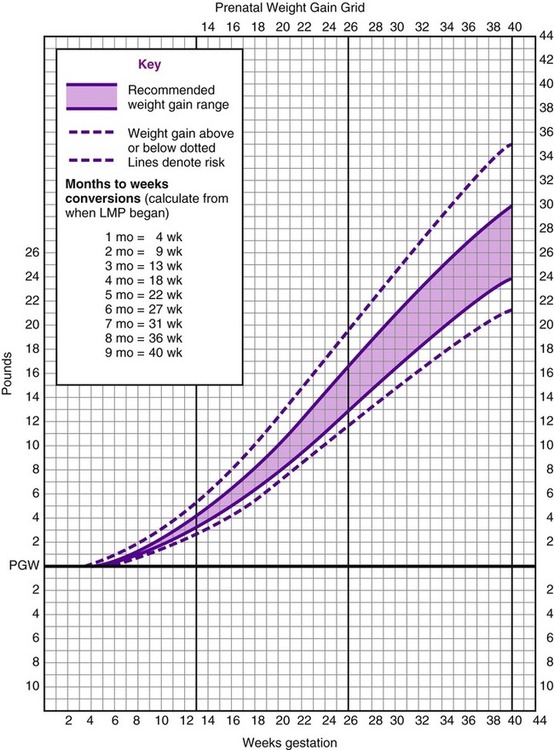
After completing this chapter, you should be able to: • Identify nutritional needs during pregnancy, lactation, and infancy. • Discuss lactation management techniques. • Discuss infant feeding strategies. • Identify risk factors and prevention of postpartum depression. Growth of the fetus (the unborn baby; Box 11-1) may be affected by various maternal factors, for example, the ingestion, digestion, absorption, and metabolism of nutrients. The fetus is dependent on these processes and transfer of nutrients through the placenta (the organ that allows the transfer of maternal nutrients to the fetus via the umbilical cord—referred to as the afterbirth at the time of delivery). An intact placenta of good size is critical for ideal growth of the fetus. To promote healthy outcomes during pregnancy the American Dietetic Association advises optimal weight gain; physical activity as appropriate; a varied diet that follows the 2005 Dietary Guidelines for Americans; vitamin and mineral supplementation appropriate to needs; avoidance of alcohol, tobacco, and drugs; and safe food handling. Women who are not meeting these goals or have chronic health problems can benefit from a referral to a registered dietitian (Kaiser and colleagues, 2008). A well-balanced diet will help the fetus grow well and allow the mother to stay healthy for future pregnancies. Table 11-1 shows a sample menu to promote a healthy pregnancy. Table 11-1 Sample Meal Plans for Pregnancy *Needs more kcalories, protein, and calcium. †For women with gestational diabetes, juice may be deemed inappropriate for control of blood glucose; oranges or other vitamin C–containing fruit may be advised later in the day rather than at breakfast, and desserts should be restricted based on values obtained from the woman’s self-monitoring of blood glucose levels (SMBG). A major determinant of fetal outcome during pregnancy is maternal weight gain. Adequate weight gain improves fetal growth. A woman who is underweight before pregnancy may benefit with more weight gain than is typically recommended to best promote development of the placenta and vasculature needed to deliver nutrients to the fetus. However, excess weight gain needs to be avoided for the health of the mother and the growing fetus. The weight gain advised for overweight women is for the products of conception (Figure 11-1). • 25 to 35 lb for normal-weight women (body mass index [BMI] 20 to 26) • 28 to 40 lb for an underweight woman (BMI < 20) Average weight gain of about 1 lb per week in the second and third trimesters of pregnancy is expected. A grid can be used to plot weight gain throughout the pregnancy (Figure 11-2). However, current evidence is once again suggesting lower levels of weight gain during pregnancy are optimal. Evidence supports the Institute of Medicine (IOM) weight gain recommendation that will help to achieve better pregnancy outcomes in obese and overweight women (Jain and colleagues, 2007). Some distinctions with the new 2009 IOM guidelines are as follows: • 28 to 40 lb for underweight women (BMI < 18.5) • 25 to 35 lb gain for normal-weight women (BMI 18.5–24.9) • 15 to 25 lb for overweight women (BMI 25–29.9) Only about one third of U.S. women gain within IOM recommendations, with most far exceeding the goals (Olson, 2008). A large proportion of adolescents gain more than is recommended by the IOM. However, the use of adult BMI categories for determining weight gain in adolescent pregnancies may not be appropriate (Groth, 2007). For multiple births increased weight gain is expected. One study of twins found the optimal rates of fetal growth and birth weights were associated with the maternal weight gains listed in Table 11-2 (Luke and colleagues, 2003). Table 11-2 Prenatal Weight Gain Recommendations for Multiple Birth For triplet gestations a higher weight gain would be expected. A normal prepregnancy BMI and a total gestational weight gain of at least 35 to 45 lb are associated with fewer pregnancy complications (Eddib and colleagues, 2007). To promote a healthy diet, a pregnant woman should be encouraged to consume at least the minimum number of servings recommended by the 2005 Dietary Guidelines and the MyPyramid Food Guidance System (Table 11-3), with a focus on the use of whole grains and unprocessed or minimally processed foods. This equates to about 1500 kcal daily depending on choices. This is likely too few kcalories, but if weight goals are being met and there are no ketones (see section on diabetes), this level may be appropriate. Table 11-3 Changes in Foods From the MyPyramid Food Groups During Pregnancy and Lactation If fortified milk is not used, obtain physician’s instructions for vitamin D supplementation. Use water or other beverages—at least 6 to 8 c daily. *Additional servings of these or any other food may be added as needed to provide the necessary calories and palatability. †Total vegetable and fruit intake advised to be at least Revised weight gain goals are being developed, in part, because of the increased numbers of large-size babies. Macrosomia (birth weight more than 4 kg or 8.8 lb) is associated with problems for both mother and infant. Achieving appropriate weight gain appears to be the most important factor in preventing macrosomia rather than the woman’s initial BMI (Kabali and Werler, 2007). One study found women who gain more than recommended by the IOM are three times more likely to have an infant with macrosomia (Hedderson and colleagues, 2006). At increased risk of having an infant with macrosomia are women who have conditions related to the metabolic syndrome, such as high BMI, excess pregnancy weight gain, and type 2 or gestational diabetes mellitus (GDM) (Henriksen, 2008). Low level of pregestational physical activity also increases risk of macrosomia (Voldner and colleagues, 2008). Among a population of white women the strongest single predictors for macrosomia were history of having a baby with macrosomia, BMI greater than 23, and prior GDM (Ogonowski and colleagues, 2008). Uncontrolled diabetes results in increased availability of glucose, which promotes fetal insulin secretion and fetal growth. However, it has been found that even with good blood glucose management, macrosomia can still occur. This may be due to increased amounts of amino acids that are delivered to the fetus. In uncontrolled diabetes during the first trimester even short intervals of poor control can adversely affect placental growth and transport function for the remainder of pregnancy, thereby contributing to macrosomia (Jansson and colleagues, 2006). Vaginal delivery of a macrosomic fetus requires an experienced obstetrician because of concerns of shoulder dystocia (a condition in which the newborn’s head is normal size, but with large shoulders) making vaginal delivery extremely difficult, and that can lead to newborn asphyxia. A macrosomic infant greater than 4 kg is the borderline birth weight at which there is increased frequency of birth canal injuries (Hirnle and colleagues, 2007). The impact of macrosomia can lead to adult health problems for the child. One study found older children who had been large for gestational age at birth and exposed in utero to the mother’s diabetes or maternal obesity had evidence of metabolic syndrome as measured by a fasting glucose/insulin ratio less than 7 (Boney and colleagues, 2005). There has been an upsurge in recent years of prematurity and low birth weight (LBW) (less than 5.5 lb) of unknown etiology. One possible cause is reduced milk intake. Women who consume less than three glasses of milk per day have been noted to have increased risk of having infants who are born small for gestational age (SGA) (less than 10th percentile height or weight based on gestational age) (Olsen and colleagues, 2007). Iron supplementation in pregnant women even without anemia is associated with reduced risk of having LBW infants (Palma and colleagues, 2008). One goal in the prevention of SGA infants is related to cholesterol levels later in childhood. Infants who were born SGA had increased cholesterol levels at age 5 years (Ogden and colleagues, 2008). There also is some evidence that intrauterine growth retardation (IUGR) or low birth weight is significantly associated with low-normal kidney function in adulthood, and more so in men (Hallan and colleagues, 2008). Hydrogenated, or trans fatty acids, should be limited in general, but especially during pregnancy. In a rat study it was found hydrogenated vegetable fat consumed during gestation and lactation alters the blood lipid profiles and promotes inflammation in the offspring (Pisani and colleagues, 2008). In another study, rats exposed to trans fats via hydrogenated soybean oil were shown to have altered appetite regulation and insulin receptors, suggesting intrauterine programming for risk of later development of obesity and hyperglycemia (Albuquerque and colleagues, 2006). Although a woman’s ability to absorb minerals is enhanced during pregnancy, an adequate dietary intake is still best. Vitamin supplements should be used only as added insurance, not as a replacement for nutrients found in foods. In fact, common prenatal multivitamins do not have choline and are low in magnesium and zinc content. Supplements should not exceed the DRIs (see the back of the book). Minerals such as zinc, copper, and magnesium are found in whole grains and legumes. Adequate consumption of dark green, leafy vegetables (folic acid) and deep orange vegetables and fruits should be encouraged for a source of beta-carotene (vitamin A). Vitamin C foods, such as citrus fruits and dark green, leafy vegetables, should be increased during pregnancy and lactation. Milk is also an important contributor to a healthy pregnancy. It is a source of calcium, vitamin D, and even magnesium and potassium. It has been found that the intake of magnesium, potassium, and folate during pregnancy is related to total body bone mineral content of the children at 9 years of age (Tobias and colleagues, 2005). Good nutritional status for an optimal pregnancy outcome begins long before conception. Adequate growth and development (see Chapter 12) of the female child helps support reproductive health. Having adequate nutrient stores to allow for fetal growth and development is critical. There is an increased need for folic acid supplementation with several health risks. This includes epilepsy, type 1 diabetes, class II or III obesity (see Chapter 6), family history of neural tube defect, and belonging to a high-risk ethnic group, such as the Sikh population. A higher intake also is advised for women with a history of poor compliance, variable diet, no consistent birth control, and substance use. The recommended intake for high-risk women is a multivitamin with 5 mg folic acid, beginning at least 3 months before conception and through the first semester of pregnancy. During the second and third trimesters of pregnancy, the postpartum period, and throughout breastfeeding 0.4 to 1.0 mg is advised (Wilson and colleagues, 2007). Folate intake by women of childbearing age in the United States has decreased by approximately 130 mcg/day, after an earlier rise from food fortification. This is believed to be due to lower-carbohydrate diets and consequently less intake of fortified grain products. There are concerns of an increased incidence of neural tube defects, and recommendations have been made to consider the optimal means to fortify the food supply (Quinlivan and Gregory, 2007). There may be other factors required to reduce the incidence of neural tube defects that are related to homocysteine. Adequate intake of vitamins B12 and B6 is advised for all women of reproductive age (Candito and colleagues, 2008). Without these B vitamins a number of biologic processes are affected as a result of suppressed methylation (addition of CH3 to various body compounds). Due to its short half-life, vitamin B6 is particularly important for undisturbed embryo (the term used for the fetus in the first trimester) development. It should be taken along with folic acid as a periconceptional supplement to prevent embryonic deformities (Weingärtner and colleagues, 2007). Vitamin B12 deficiency, such as found with a vegan diet or among women with previous bariatric weight loss surgery, should be considered for infants with encephalopathies (degenerative diseases of the brain) that may be reversible if caught early (Gutiérrez-Aguilar and colleagues, 2005). Obesity is an epidemic worldwide, and its adverse effects on conception, the health of the fetus, and lactation are now being recognized. Maternal risks of obesity during pregnancy include gestational diabetes, hypertension and preeclampsia (see later section), and increased incidence of delivery complications, whereas risks to the fetus include miscarriage, macrosomia, and stillbirth (Catalano, 2007). The ability to successfully breastfeed also is impaired with obesity. Increased efforts are likely required to promote the production and flow of breast milk with an obese woman. Women should ideally be counseled to prevent obesity or lose weight before conception. Bariatric weight loss surgery is increasingly being advocated for women of childbearing years. This can help in some regards, but other adverse effects can occur with these procedures. Extremely obese persons often have nutritional deficiencies, particularly in fat-soluble vitamins, folic acid, and zinc. Bariatric surgery can worsen these deficiencies because of the severely reduced food portions and malabsorption in bypass procedures. Protein deficiency can develop that may require enteral or parenteral nutritional support (see Chapter 15). Anemia can develop from deficiency of iron, folic acid, and/or vitamin B12. Wernicke encephalopathy from thiamin deficiency can develop that causes neurologic damage. A thorough nutritional follow-up should be performed before conception and during pregnancy after obesity surgery (Folope, Coëffier, and Déchelotte, 2007). It is paramount, however, that tight control over blood sugar levels be achieved before conception to help prevent birth defects from developing during the critical first trimester. For women with preexisting type 2 diabetes, the same precautions need to be taken. With uncontrolled type 2 diabetes, rates of perinatal mortality (25 per 1000) and congenital malformation (99 per 1000) are at least as poor as those in uncontrolled type 1 diabetes. The rates of hypertension, preeclampsia, cesarean birth, and postpartum hemorrhage are increased with uncontrolled type 2 diabetes (Dunne, 2005). An added concern of women who are taking insulin to manage their diabetes is nighttime hypoglycemia. Lantus has not yet been approved for the management of diabetes because of its altered protein structure. Lantus would be the optimal long-acting insulin to use because it is peakless with low likelihood of hypoglycemia. Until Lantus is found to be safe to use during pregnancy, the other long-acting insulin, NPH, is advised. However, NPH does increase the risk of hypoglycemia, especially during the time of peak action, which is about 6 hours, with a variable range of peak action between 4 and 8 hours. It is for this reason that evening doses of long-acting insulin should be given at bedtime to allow for the peak action to occur closer to the time of the dawn phenomenon after 4 AM (refer to Chapter 8). An evening snack that includes carbohydrate is recommended for all women during pregnancy to decrease production of ketones, but such a snack will also help prevent nighttime hypoglycemia. With the advent of insulin pump therapy increased numbers of women during pregnancy use this technology. With appropriate and educated use of insulin pumps, maintaining normal blood glucose levels is easier than provision of insulin by injection. However, risks still exist. In one study the rates of diabetic ketoacidosis (DKA) and neonatal hypoglycemia were significantly higher in the group using pump therapy (Chen and colleagues, 2007). Neonatal hypoglycemia needs to be avoided as much as hyperglycemia. Maintaining normal glucose levels during fetal life depends entirely on continuous placental glucose transfer. If the maternal level of blood glucose is low, the placental transfer of glucose to the fetus will be low. A number of health concerns for the fetus can develop with exposure to hypoglycemia. One problem is seizures (Okanishi and colleagues, 2008). Hypoglycemia is linked with developmental delay and permanent brain damage. Intrauterine growth retardation and prematurity may also be a consequence of fetal hypoglycemia. Hyperglycemia during pregnancy causes the fetus to increase endogenous insulin production, which can lead to hypoglycemia when this high supply of glucose is stopped, such as at delivery, but also potentially in utero. The goal of treatment in infants with hyperinsulinemia is to prevent brain damage from hypoglycemia by maintaining plasma glucose at 70 mg/dL (Palladino, Bennett, and Stanley, 2008). Control of pregnancy with diabetes is best handled with a medical team approach, so that the most appropriate plan and means of control are developed, including aspects such as insulin, diet, and home glucose monitoring. This includes the mother in making decisions regarding how to manage her blood glucose levels. Discussion should include the benefits of diabetes management during pregnancy. This has been shown to help compliance and outcomes with a group of educated women realizing good metabolic control (A1c of 7.5%; see Chapter 8) as compared with a noneducated group (A1c of 8.4%). Clinical outcomes noted in the group of educated women included control of retinopathy, milder cases of preeclampsia, and no perinatal deaths (Todorova, Mazneikova, and Ivanov, 2004). Although this study defined good control with an A1c of 7.5%, most health care professionals advise an A1c in the 5% range during pregnancy for optimal fetal outcomes. There are different viewpoints on optimal maternal blood glucose levels. The American College of Obstetricians and Gynecologists (ACOG, 2005) goals are as follows: • Less than or equal to 95 mg/dL fasting • Less than or equal to 100 mg/dL preprandial • Less than or equal to 140 mg/dL 1-hour postprandial The American Diabetes Association (ADA, 2004) advises the following: Development of certain conditions have been related to season of birth, including schizophrenia, multiple sclerosis, type 1 diabetes, and longevity. This may be influenced by seasonal variation in nutrient intake during pregnancy (Watson and McDonald, 2007). Significantly fewer adolescents living in Chicago who developed diabetes were born during October. This was especially true among males and those who apparently had developed type 2 diabetes (Grover, Lipton, and Sclove, 2004). One nutrient that is influenced by season is vitamin D. This is certainly true in Northern climates, but there is evidence that subclinical vitamin D deficiency can occur, even in regions with a temperate climate at a latitude 39.5 degrees N (Cabezuelo and colleagues, 2007). Vitamin D deficiency in utero has been linked with craniotabes (soft bone tissue of the skull due to reduced mineralization) in otherwise normal neonates. The condition can persist at least until 1 month of age among breastfeeding infants (Yorifuji and colleagues, 2008). The quality of male sperm is now recognized as being important in achieving a healthy pregnancy outcome. Even moderate increases in blood lead content were found to be related to an increase in immature sperm concentration, and altered size of sperm (Telisman and colleagues, 2007). Cigarette smoking increases levels of both lead and cadmium in semen and leads to increased oxidative damage to sperm (Kiziler and colleagues, 2007). Vitamin C has been linked with healthier sperm. A low level of seminal ascorbic acid is associated with DNA damage of sperm. An adequate intake of vitamin C helps prevent oxidative damage of sperm quality (Song, Norkus, and Lewis, 2006). The plasma ascorbic acid content of semen was found decreased in smokers and in infertile men. Beyond DNA damage of sperm, low vitamin C levels and smoking are related to low sperm count and motility (Mostafa and colleagues, 2006). As reviewed previously in the book, excess vitamin C is linked with increased oxidative damage. The conception of a healthy pregancy may be helped when men consume moderate amounts of a variety of vitamin C foods. Good nutritional status begins before conception and can help prevent birth defects. Down syndrome may be influenced by maternal nutritional status. Children with Down syndrome have been noted to have abnormalities in the metabolism of vitamin B6. Oxalate is a marker of pyridoxine deficiency and is elevated in the amniotic fluid of fetuses with Down syndrome (Baggot and colleagues, 2008). Once a pregnancy has been successfully conceived, the first trimester is the critical period of pregnancy. This is the period during which the embryo develops (see Box 11-1). In this period all formation of organs occurs, such as that of the heart, brain, liver, and intestinal tract. Iron deficiency anemia may be evident at the start of pregnancy. This can be due to a variety of problems. For any woman during pregnancy, iron deficiency anemia may occur later during pregnancy when iron intake and stores do not meet increased demands. This is generally preventable and treatable by daily supplements of 30 to 60 mg of ferrous salts. For women who do not tolerate large doses of iron, 18 mg is the standard amount in multivitamin and mineral preparations. However, evidence suggests intolerance to iron supplements is due to tablet size, rather than the iron content (Nguyen and colleagues, 2008). An increased intake of red meat and cereals fortified with iron (in combination with a vitamin C source) may be adequate to meet iron goals. Undertaking iron studies is advised if there is concern about iron deficiency anemia. This is, in part, due to potential for iron overload. Endometriosis, for example, appears to be adversely affected by iron overload (Defrère and colleagues, 2008). A newer cause of anemia is related to bariatric weight loss surgery, which is increasingly being performed on women of childbearing years. Iron deficiency develops after gastric bypass for several reasons, including intolerance of red meat, diminished stomach secretions, and malabsorption with removal of portions of the small intestine. Oral iron supplementation and vitamin C in addition to a multivitamin should be prescribed for women of childbearing years who have had bariatric surgery. Monitoring is required because it may take years before anemia develops. Once iron deficiency has developed, parenteral iron or blood transfusions may be required (Love and Billett, 2008). Other forms of anemia also may occur during pregnancy. Copper deficiency causes one form of anemia. Both iron and copper are essential micronutrients and are required for a wide variety of enzymatic and other processes within the developing fetus (McArdle and colleagues, 2008). Deficiency of vitamin B12 or folic acid, both forms of macrocytic anemia, may be a cause. If vitamin B12 deficiency is suspected during pregnancy, as with a woman following a vegan diet, urinary methylmalonic acid excretion and plasma homocysteine levels should be determined and vitamin B12 supplementation should be started during pregnancy as needed and with the young infant to avoid potentially irreversible damage of the fetus and infant. One case of severe vitamin B12 deficiency involved a 7-month-old infant who developed muscular weakness believed to be due to impaired myelination that was irreversible (Schlapbach and colleagues, 2007). Preliminary evidence suggests ginger (Zingiber officinale) may be an effective and safe treatment for nausea and vomiting in pregnancy. Use of 650 mg of ginger or 25 mg of vitamin B6 3 times per day were both found to be effective for treatment of nausea and vomiting in pregnancy. However, ginger was more effective than vitamin B6 (Chittumma, Kaewkiattikun, and Wiriyasiriwach, 2007). Doses needed for control of nausea and vomiting have not been specifically determined. One study found the dose of 1 g of ginger or 40 mg of vitamin B6 daily were equally effective in reducing vomiting, and once again ginger was found to be more effective in controlling nausea (Ensiyeh and Sakineh, 2008). Applied in daily doses of up to 6 g, fresh ginger root generally has few side effects (Betz and colleagues, 2005). The level of vitamin B6 in treating nausea and vomiting is much higher than the DRI. The average dose of vitamin B6 in one study, approximately 130 mg/day for a period of about 5 to 13 weeks, was associated with one major malformation, and the average birth weight was approximately 3000 to 4000 g (Shrim and colleagues, 2006). Long-term ingestion of vitamin B6 greater than 100 mg/day is associated with neuropathy. The DRI for vitamin B6 is 2.6 mcg during pregnancy, and the upper tolerable intake level (UL) has not been determined. Caution is advised. • Eat frequent, small meals (93% of providers) • Snack on soda crackers (68.5%) • Take vitamin B6 along with medication (67.1%) (Power, Milligan, and Schulkin, 2007) Hyperemesis gravidarum, commonly referred to as simply hyperemesis, is characterized by excessive and prolonged vomiting beyond the first trimester. The severity of vomiting leads to significant weight loss, and the diagnosis includes a weight loss of over 5% with electrolyte imbalance. It is probably more common than statistics show because treatment may be in a physician’s office rather than in a hospital setting. The cause of hyperemesis is generally unknown, although various issues have been implicated, including hyperthyroidism, infection with Helicobacter pylori, and altered hormones. Hyperemesis can cause serious dehydration and vitamin deficiencies, such as thiamin deficiency, which causes signs and symptoms of beriberi and Wernicke’s encephalopathy (Indraccolo and colleagues, 2005). It is advised to check for ketones (see section on gestational diabetes) in order to determine management need (Sheehan, 2007). Control of ketones requires hydration and insulin, but with hyperemesis of pregnancy provision of carbohydrates also will be required. An assessment of treatment of women who had suffered with hyperemesis for at least 27 weeks revealed the most effective treatments included intravenous (IV) hydration, serotonin inhibitors, and parenteral nutrition (Goodwin and colleagues, 2008). However, parenteral nutrition (provision of nutrients directly into large veins; see Chapter 15) is not without risk. One study found peripherally inserted central catheter (PICC; see Chapter 15) line has the potential for greater complications, such as infection and clot formation, while providing no advantage over tube feeding (see Chapter 15) in the management of pregnancies with hyperemesis (Holmgren and colleagues, 2008). On a positive note, the inclusion of omega-3 fatty acids is now recognized to promote the development of the neurologic system in utero and early infancy. The primary source of omega-3 fatty acids, fish, comes at the risk of mercury poisoning. Sources relatively low in mercury include salmon, followed by shrimp, as the prime sources of omega-3 fatty acids. The more popular tuna can be consumed for omega-3 fatty acids but poses a greater risk for mercury toxicity (Mahaffey, Clickner, and Jeffries, 2008). One debate is how much fish is enough to provide the benefits of the omega-3 fatty acids without being detrimental due to mercury content. Higher fish intake was found associated with better child cognitive test performance, but higher mercury levels with poorer test scores. Fish consumption of two servings or less per week was not associated with a benefit (Oken and colleagues, 2008). Certain nutrients have greater effects on brain development than do others. These include protein, energy, certain fats, iron, zinc, copper, iodine, selenium, vitamin A, choline, and folate. Deficiency of these nutrients during fetal development includes adverse effects on information processing, memory, autonomic nervous regulation, and motor coordination (Georgieff, 2007). Adequate intake of the B vitamins during pregnancy is important to promote neurologic development of the fetus. A dietary deficiency of vitamin B1 is a cause of nerve damage. Wernicke’s encephalopathy can occur with severe hyperemesis. Thiamin supplementation should be provided to women with prolonged vomiting in pregnancy, especially at the first indication of neurologic signs and symptoms (see Chapter 3) (Chiossi and colleagues, 2006). Optimally thiamin should be provided intravenously in such a situation. Vitamin B12 deficiency in utero (and in infancy) causes growth retardation, impaired or regression of psychomotor development, muscular hypotonia, and brain atrophy (Lücke and colleagues, 2007). During gestation there is a high demand for the B vitamin choline. Adult rats supplemented with choline during early gestation have improved memory performance throughout their life span, whereas prenatally choline-deficient rats have memory deficits (Kovacheva and colleagues, 2007). It has been demonstrated that choline has a lasting effect on brain and behavior of the rat offspring (Cheng and colleagues, 2008). In one case of depletion of plasma choline and a similar substance in a pregnant woman, low placental weight and low infant birth weight was noted. The cause of the choline depletion appeared related to medications used to treat her bipolar disorder (Gossell-Williams, Fletcher, and Zeisel, 2008). Another B vitamin, pyridoxine, affects development of the part of the brain related to the learning process and memory retention. Reduced intellect and altered behaviors may occur in children of women with pyridoxine deficiency during pregnancy. Deficiency of this vitamin is common among women who used anovulatory steroids before pregnancy (Krishna and Ramakrishna, 2004). Neurologic development continues into the second trimester and throughout early infancy. For women with low intake of fish, a daily vitamin/mineral supplement containing 200 mg docosahexaenoic acid (DHA) appears appropriate. This level of DHA given beginning at the twenty-first week of gestation significantly raised maternal levels of DHA at both the thirty-seventh week and 3 weeks postpartum, with breast milk containing twice the level of DHA as for women without the supplement (Bergmann and colleagues, 2008). GDM occurs after the placenta has reached a large enough size to release significant amounts of placental hormones into the mother’s circulatory system. These hormones worsen insulin resistance and lead to high blood glucose levels, especially for women who already have a predisposition toward insulin resistance. The first indication of metabolic syndrome may be the diagnosis of gestational diabetes (Kaaja, 2008). An estimated 4% of pregnancies are GDM pregnancies. Debate continues on how best to screen for GDM and when to treat. Routine screening for GDM is now generally done between the twenty-fourth and twenty-eighth weeks of pregnancy. A 1-hour screening with a 50-g oral glucose challenge test is advised for high-risk women. This includes women who are obese or 32 years of age or older (Hackmon and colleagues, 2007). If the initial 1-hour screening level is greater than or equal to 140 mg/dL, a 3-hour oral glucose tolerance test (OGTT) is advised, using a 100-g glucose drink. Having two of the following values above normal is diagnostic of GDM: Whether a woman with impaired glucose tolerance according to the results of an OGTT, but not diagnosed with GDM, should monitor her blood glucose levels during pregnancy is not agreed upon. One study found women with one elevated oral glucose tolerance test value did not benefit from medical nutritional therapy and self-monitoring of blood glucose (Fassett, Dhillon, and Williams, 2007). However, more than one third of mothers who have delivered at least one large baby have been found to have had impaired glucose tolerance, based on A1c (see Chapter 8), after delivery. An elevated prevalence of preeclampsia with impaired glucose metabolism during pregnancy also has been noted. Prevention of excess weight gain is important among women who have an abnormal OGTT result but do not meet the diagnostic criteria for GDM because they are at increased risk of delivering macrosomic and large-for-gestational-age neonates (Segal and colleagues, 2008). Controversy also continues on how best to treat GDM. The controversies in GDM management include how low kcalorie intake should be, dietary composition of carbohydrates versus fats, and optimal gestational weight gain. There is evidence that the omega-3 fatty acid DHA reduces insulin resistance. Indications that food restrictions have gone too far include weight loss or lack of weight gain, undereating to avoid insulin therapy, positive urinary ketones, and intentional restriction of healthy foods (Reader, 2007).
Maternal and Infant Nutrition in Health and Disease
INTRODUCTION
PREGNANT WOMAN
PREGNANT ADOLESCENT
Breakfast*
Orange juice,† 1 c
Orange juice,† 1 c
Shredded wheat
Shredded wheat
Scrambled egg
Scrambled egg
Toast, 1 slice
Toast, 2 slices
Milk, 1 c
Butter or margarine
Decaffeinated coffee
Marmalade†
Milk, 1 c
Lunch
Tuna sandwich on whole-grain bread
Tuna sandwich on whole-grain bread
Carrot and green pepper sticks
Carrot and green pepper sticks
Oatmeal cookies†
Cheese cube
Milk, 1 c
Oatmeal cookies†
Fresh fruit
Milk, 1 c
Midafternoon snack
Milk, 1 c
Chicken sandwich
Milk, 1 c
Dinner
Broiled steak
Broiled steak
Steamed broccoli
Steamed broccoli with melted cheese
Baked potato
Baked potato with sour cream
Tomato salad with French dressing
Vegetable salad with French dressing
Apple slices
Apple with peanut butter
Milk, 1 c
Bedtime
Hot milk or cocoa,† 1 c
Milk or cocoa,† 1 c
WHAT GENERAL NUTRITIONAL ADVICE IS RECOMMENDED DURING PREGNANCY?
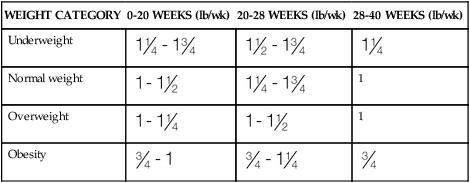
WEIGHT GAIN
WEIGHT CATEGORY
0-20 WEEKS (lb/wk)
20-28 WEEKS (lb/wk)
28-40 WEEKS (lb/wk)
Underweight
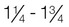
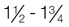
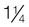
Normal weight
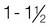
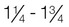
1
Overweight
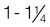
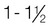
1
Obesity
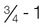
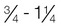
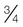
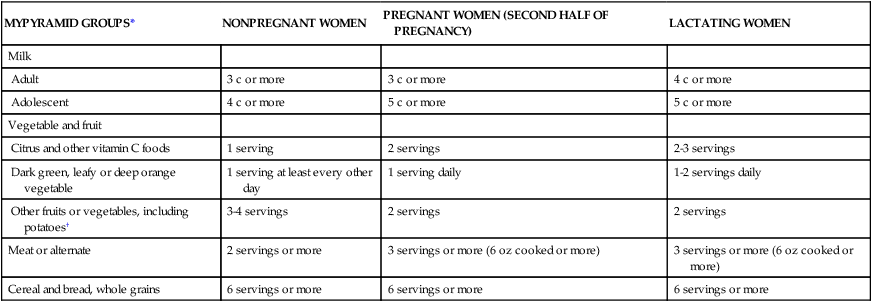
MYPYRAMID GROUPS*
NONPREGNANT WOMEN
PREGNANT WOMEN (SECOND HALF OF PREGNANCY)
LACTATING WOMEN
Milk
Adult
3 c or more
3 c or more
4 c or more
Adolescent
4 c or more
5 c or more
5 c or more
Vegetable and fruit
Citrus and other vitamin C foods
1 serving
2 servings
2-3 servings
Dark green, leafy or deep orange vegetable
1 serving at least every other day
1 serving daily
1-2 servings daily
Other fruits or vegetables, including potatoes†
3-4 servings
2 servings
2 servings
Meat or alternate
2 servings or more
3 servings or more (6 oz cooked or more)
3 servings or more (6 oz cooked or more)
Cereal and bread, whole grains
6 servings or more
6 servings or more
6 servings or more
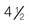 c daily.
c daily.
MACROSOMIA
LOW BIRTH WEIGHT
MACRONUTRIENTS
MICRONUTRIENT NEEDS
HOW DOES NUTRITION INFLUENCE THE OUTCOME OF PREGNANCY?
PREGNANCY PLANNING
PREVENTION OF NEURAL TUBE DEFECTS
OBESITY
PREEXISTING DIABETES MANAGEMENT
IMPACT OF SEASON
INFLUENCING CONCEPTION
PREVENTION OF BIRTH DEFECTS
WHAT ARE NUTRITIONAL CONCERNS THROUGHOUT THE TRIMESTERS OF PREGNANCY?
FIRST-TRIMESTER CONCERNS
Anemia
Morning Sickness
Hyperemesis Gravidarum
Promoting Neurologic Development
SECOND-TRIMESTER CONCERNS
Physiologic Anemia
Screening for and Management of Gestational Diabetes Mellitus (GDM)
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Maternal and Infant Nutrition in Health and Disease


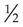 -cup scoop of ice cream is on average 170 kcal. The Dietary Reference Intake (DRI) should be met at 130 g carbohydrates, with additional quantities to prevent ketone formation and to promote weight gain needs. Carbohydrate intake should be spread over the day with a maximum of 10 hours between meals. This means a bedtime snack and breakfast containing carbohydrate is important, along with the main mealtimes of lunch and supper.
-cup scoop of ice cream is on average 170 kcal. The Dietary Reference Intake (DRI) should be met at 130 g carbohydrates, with additional quantities to prevent ketone formation and to promote weight gain needs. Carbohydrate intake should be spread over the day with a maximum of 10 hours between meals. This means a bedtime snack and breakfast containing carbohydrate is important, along with the main mealtimes of lunch and supper.
