Mastectomy has been utilized in the surgical management of patients with breast cancer for centuries. It was William Stewart Halsted who popularized the technique of radical mastectomy after his initial publication in the mid-1890s.1 Halsted believed that breast cancer spread from the primary tumor in the breast parenchyma to the regional lymph node basins and then to distant sites. He felt that this sequential progression of spread could be halted if all of the breast tissue, skin, chest wall musculature, and regional lymphatics were resected. In addition to the extensive chest wall resection, the Halsted radical mastectomy included removal of the level I, II, and III axillary lymph nodes. Halsted’s initial publication reported a 5-year survival rate of 40% and a local-regional control rate of 73%. The extended radical mastectomy was introduced to include internal mammary nodal dissection based on retrospective comparisons showing improved survival with the more extensive procedure. A multinational randomized trial was initiated in the 1960s to compare survival rates with the Halsted radical mastectomy versus the extended radical mastectomy.2 The trial did not show any difference in survival outcomes between the two surgical procedures, however it was underpowered and patients were not staged and selected for participation based on imaging studies. Subsequent studies failed to confirm any survival advantage with the extended radical mastectomy and this procedure was largely abandoned in favor of radiation to the regional nodes.
In 1943, Haagensen and Stout first introduced the criteria for inoperability of patients with advanced breast cancer who would not be expected to have a survival benefit from the radical mastectomy.3 These included inflammatory carcinoma, evidence of satellite skin nodules or extensive edema of the skin of the breast, ulceration or fixation of the tumor to the chest wall, and fixed axillary lymph nodes. They suggested that operative management of patients with these findings was not beneficial and did not result in local-regional control or long-term survival. It was not until the introduction of effective systemic therapies and the incorporation of radiation into the local-regional management of breast cancer that survival outcomes improved in patients with locally advanced disease. Multidisciplinary management of patients with advanced breast cancer remains the standard of care today.
Although some surgeons began to explore less radical approaches to the surgical management of breast cancer in the early 1900s, the Halsted radical mastectomy remained as a standard well into the 1970s even for patients with early-stage breast cancer. The modified radical mastectomy was introduced as an alternative to radical mastectomy and included removal of the breast and level I, II, and III axillary lymph nodes but preserved the pectoralis major muscle. Murphy and colleagues4 were proponents of preservation of the pectoralis muscles during mastectomy as early as 1912. Patey and Dyson5 modified the technique of radical mastectomy for patients with early-stage breast cancer by preserving the pectoralis major and removing the pectoralis minor muscle to facilitate a complete level I, II, and III axillary lymph node dissection.
In fact, it was not until randomized trials were published comparing the radical mastectomy with less aggressive approaches that the use of radical mastectomy fell out of favor. Initially there were some small studies comparing radical mastectomy and modified radical mastectomy that did not report any survival differences. The landmark National Surgical Adjuvant Breast and Bowel Project (NSABP) B-04 trial was the first large randomized trial comparing radical mastectomy with total mastectomy with regional nodal radiation versus total mastectomy alone for patients with clinically node negative breast cancer.6 Patients in the mastectomy alone arm only had treatment of the axillary nodes if they developed clinical evidence of axillary metastasis. The initial publication of B-04 and a subsequent publication with 25 years of follow-up did not show any survival differences between the treatment arms. The overarching hypothesis was that the type of local-regional therapy did not impact breast cancer survival and challenged the Halsted concept of sequential spread of cancer cells from the breast to the regional nodes and then to distant sites. Fisher’s “alternative hypothesis” was that breast cancer cells had access to the systemic circulation at initial diagnosis and the choice of local therapy did not impact survival outcomes. Following publication of the NSABP B-04 trial, several large randomized trials were initiated comparing breast-conserving surgery and radiation as an alternative to mastectomy in the treatment of patients with early-stage breast cancer.
During the 1970s and 1980s there was a gradual transition from the Halsted radical mastectomy to the modified radical mastectomy. The use of adjuvant radiation therapy and systemic chemotherapy were incorporated into the treatment paradigm as evidence emerged from clinical trials and the Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) was established to evaluate the results of randomized clinical trial data with respect to the impact of adjuvant therapy on breast cancer recurrence and mortality. There is now mature data available from the EBCTCG overview analysis demonstrating the importance of systemic therapy in decreasing recurrence and improving survival.7 The overview analysis has also shown the importance of radiation therapy in reducing local-regional recurrence following mastectomy in patients with node positive breast cancer.8 The standard recommendation is for use of postmastectomy radiation therapy in patients with four or more positive axillary nodes and for patients with stage III breast cancer. The most recent overview analysis suggests a benefit in terms of reduced recurrence and improved survival in patients with one to three positive lymph nodes. It is important to consider the need for postmastectomy radiation in surgical treatment planning as decisions regarding breast reconstruction can impact the ability to deliver radiation and the final cosmetic outcome. The use of breast reconstruction and the sequencing of radiation and breast reconstruction will be discussed later in this chapter.
Selection of patients for mastectomy depends on multiple patient and tumor-related factors. Modified radical mastectomy is largely performed for patients with known node positive disease and those with more locally advanced breast cancer that requires significant skin resection. The introduction of sentinel lymph node biopsy for regional nodal staging has eliminated the role for axillary lymph node dissection in many patients with early-stage breast cancer. However, some patients with early-stage breast cancer will choose mastectomy over breast-conserving surgery and a complete discussion of the pros and cons of mastectomy should be undertaken. Individuals who have a genetic predisposition to developing breast cancer (BRCA mutation carriers) may be better suited for mastectomy because of the high lifetime risk of developing new primary breast cancers. Other reasons to consider mastectomy include contraindications to breast conservation such as prior radiation therapy to the breast or chest wall, multicentric disease, a history of scleroderma or lupus erythematosus, and extensive microcalcifications that would preclude complete excision with negative margins.
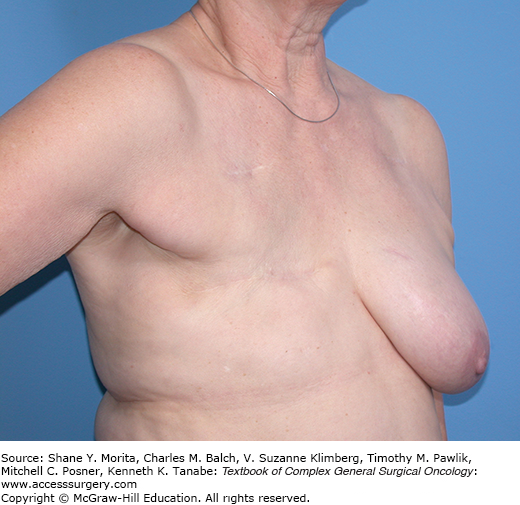
Modified radical mastectomy was originally described as complete excision of the skin of the breast to include the nipple and areola complex and extension of the incision toward the axilla to facilitate a level I, II, and III axillary lymph node dissection. An elliptical incision is measured out to incorporate the skin necessary for resection in order to facilitate closure of the skin without significant redundancy along the chest wall. In general, excisional biopsy scars should be incorporated into the skin ellipse. Core biopsy incisions can also be incorporated into the skin ellipse, however there is no data suggesting increased risk of local-regional recurrence should these biopsy sites be left intact. Skin flaps are elevated in a subdermal plane to incorporate the entire breast mound. The dissection is described as proceeding superiorly toward the clavicle, medially toward the sternum, inferiorly along the chest wall to the upper edge of the rectus sheath, and laterally to the latissimus dorsi muscle. The thickness of the skin flaps will vary according to the body habitus of the individual patient. In patients with a larger body habitus the skin flaps will tend to be thicker and it is important to identify the appropriate plane of dissection so as not to devascularize the skin flap which can lead to skin necrosis. Following development of the skin flaps the breast mound is removed from the chest wall along the pectoralis fascia. Traditionally, the plane of dissection includes the pectoralis fascia as the breast is removed from the chest wall although there is no data to show that local-regional failure rates are increased if the pectoralis fascia is not included in the dissection. For patients with locally advanced breast cancer where the tumor is approaching the chest wall musculature it may be necessary to resect a portion of the pectoralis major muscle to achieve negative margins. Figure 76-1 shows a patient following modified radical mastectomy without breast reconstruction.
For patients undergoing a modified radical mastectomy the axillary lymph node dissection is typically performed en bloc with the removal of the breast. Once the breast has been removed from the pectoralis major muscle the fascia is incised along the lateral extent of the pectoralis major muscle to expose the pectoralis minor. The fascia is incised along the latissimus dorsi muscle as the most lateral extent of the axillary dissection and the axillary vein is identified at the cephalad border of the axillary dissection. The axillary contents are then dissected from the axilla and the pectoralis minor muscle is retracted to facilitate removal of the level II axillary nodes. If a level III node dissection is indicated the pectoralis minor muscle can be divided to allow access to the level III nodes.
Seroma formation is one of the known occurrences following mastectomy that can impact the ability to proceed to adjuvant therapy. Most surgeons will place drains in the subcutaneous space and leave the drains in place until output is less than 30 mL per day. In general, one drain is placed along the chest wall and one in the axillary space when complete axillary lymph node dissection has been performed. Some centers perform a closure known as “quilting” or “axillary padding” where multiple sutures are placed along the chest wall to the subcutaneous tissue or in the axilla to prevent seroma formation. Although this may allow for omission of drain placement, limited data is available to support the efficacy of this approach.
Breast reconstruction is now considered an important part of the management of patients undergoing mastectomy. In the 1980s and 1990s breast reconstruction was often delayed due to concerns that recurrence may not be detected in a timely fashion if the breast reconstruction were in place. There were also concerns that the reconstruction may interfere with delivery of adjuvant therapy. As more patients were diagnosed with early-stage breast cancer, surgeons began to incorporate immediate breast reconstruction into the treatment paradigm. But, immediate breast reconstruction has superior outcomes to delayed breast reconstruction and has been shown to decrease the psychological impact of mastectomy. Immediate breast reconstruction also has the advantage of reducing the number of surgical procedures required for breast reconstruction and overall has been shown to reduce hospital costs.
Skin-sparing mastectomy was introduced by Toth and Lappert in 1991 as a means of preserving the breast skin “envelope” to facilitate immediate breast reconstruction.9 The concept was to remove the entire breast mound while minimizing the amount of skin excision in order to improve the cosmetic outcome. Initially skin-sparing mastectomy was described to include the nipple areolar complex and any previous biopsy sites with preservation of the inframammary fold. Incisions typically were designed to encompass the nipple areolar complex with a lateral extension to facilitate axillary dissection. As experience has grown with the technique multiple incisions have been described to facilitate skin preservation depending on the breast size, the reconstructive technique, the location of the primary tumor, and the type of axillary surgery.10
The oncologic safety of skin-sparing mastectomy has been studied by many investigators. There was concern that additional breast tissue may be left behind on the skin flaps and along the inframammary fold that would increase recurrence rates. Single institution reports with relatively small numbers of patients described local recurrence rates of 4% to 7% with the use of skin-sparing mastectomy compared to rates of 4% to 10% for patients undergoing standard mastectomy without skin-sparing.11 More recently Yi and colleagues12 described a large institutional experience comparing skin-sparing mastectomy with standard mastectomy from 2000 through 2005. Patients were diagnosed with stage 0–III breast cancer and were followed for local recurrence, distant metastasis, and survival outcomes. Of 1810 patients undergoing mastectomy, 799 had skin-sparing mastectomy. At a median follow-up time of 53 months there were 119 patients (6.6%) who experienced a local, regional, or distant recurrence event. There were no differences seen in the patients undergoing skin-sparing mastectomy versus conventional mastectomy. After adjusting for clinical stage at presentation and age, there were no differences in disease-free survival outcomes between the two groups. The authors concluded that skin-sparing mastectomy was an oncologically safe technique that did not result in increased recurrence rates compared with conventional mastectomy. Lanitas et al13 published a meta-analysis of nine studies including 3979 patients undergoing mastectomy. This analysis included 1104 patients having skin-sparing mastectomy. There were no differences in local recurrence rates among patients having skin-sparing mastectomy as compared to conventional mastectomy. There were fewer distant recurrences seen in the patients having skin-sparing mastectomy, however, data regarding tumor grade was not available in all of the studies included. There have not been any randomized trials addressing the oncologic safety of skin-sparing mastectomy compared with conventional mastectomy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree