, Hans Guski2 and Glen Kristiansen3
(1)
Carl-Thiem-Klinikum, Institut für Pathologie, Cottbus, Germany
(2)
Vivantes Klinikum Neukölln, Institut für Pathologie, Berlin, Germany
(3)
Universität Bonn, UKB, Institut für Pathologie, Bonn, Germany
13.1 Prostatic Tumors
Diagnostic Antibody Panel for Prostatic Adenocarcinoma (Acinar and Ductal) and Basal Cell Carcinoma
- a.
Markers for prostatic epithelium:
PSA, PAP, NKX3.1, prostein, androgen receptors, ERG, human glandular kallikrein-2 (hK2), AMACR (p504S)
- b.
Basal cell markers:
High molecular weight cytokeratins (CK5, CK6, CK14, CK34βE12), p40, p63
Prostate specific antigen (PSA) | ||
|---|---|---|
Expression pattern: cytoplasmic | ||
Main diagnostic use | Expression in other tumors | Expression in normal cells |
Carcinoma of the prostate | Salivary duct carcinoma, small cell carcinoma | Prostatic secretory and ductal epithelium, periurethral glands, male anal glands, Skene gland |
Positive control: prostatic tissue | ||
Diagnostic Approach
Prostate specific antigen (PSA) also known as kallikrin-3 is a single chain glycoprotein and a serine protease synthesized by the epithelium of prostatic gland and secreted into prostatic ducts. Normally, protease inhibitors rapidly inactivate PSA that enter the blood circulation. PSA is one of the most specific markers for prostatic parenchyma and prostatic carcinoma. Metastatic carcinoma positive for pan-cytokeratin but negative for cytokeratins 5/7/14/20 suggests a primary prostatic carcinoma, and the expression of PSA and/or NKX3.1 will confirm the prostatic origin.
Diagnostic Pitfalls
About 10% of high-grade prostatic carcinomas are negative for PSA. In such cases, other prostate-specific markers such as NKX3.1, prostate-specific membrane antigen, prostatic acid phosphatase, and androgen receptors are useful to confirm the diagnosis. Low levels of PSA expression are reported in tumors other than prostatic carcinoma. Weak expression level of PSA is found in a subset of salivary duct carcinoma. Weak expression of PSA is also reported in small cell carcinoma and breast carcinoma in addition to endometrioid carcinoma.
Prostein (SLC45A3) | ||
|---|---|---|
Expression pattern: cytoplasmic, Golgi pattern | ||
Main diagnostic use | Expression in other tumors | Expression in normal cells |
Carcinoma of the prostate | None described | Prostatic secretory and ductal epithelium, periurethral glands, male anal glands |
Positive control: prostatic tissue | ||
Diagnostic Approach
Prostein (solute carrier family 45, type 4 (SLC45A4)) is a transmembrane transporter protein found in the Golgi apparatus of prostatic secretory epithelia. Prostein is more specific to determine a prostatic origin than PSA and slightly more sensitive. Prostein can thus be successfully used in a panel with NKX3.1 and PSA to classify metastases of unknown primary tumor or to discriminate between prostatic, urothelial, or colorectal carcinomas [1]. The loss of prostein expression is associated with unfavorable clinical course [2].
Diagnostic Pitfalls
Negativity for prostein does not rule out prostatic origin.
Prostatic acid phosphatase (PAP) | ||
|---|---|---|
Expression pattern: cytoplasmic | ||
Main diagnostic use | Expression in other tumors | Expression in normal cells |
Carcinoma of the prostate | Neuroendocrine tumors, intravascular large B-cell lymphoma | Prostatic secretory and ductal epithelium, periurethral glands, male anal glands |
Positive control: prostatic tissue | ||
Diagnostic Approach
Prostatic acid phosphatase (PAP) is an enzyme secreted by prostatic epithelium and a major component of prostatic fluid. PAP is more sensitive but less specific than PSA for prostatic glands and prostatic carcinoma. PAP can be successfully used in a panel with PSA to classify metastases of unknown primary tumor.
Diagnostic Pitfalls
Similar to PSA, PAP can be also expressed in neuroendocrine carcinomas of different origin. This feature is important for the differentiation between poorly differentiated prostatic carcinoma, prostatic carcinoma with neuroendocrine differentiation, and neuroendocrine tumors.
Androgen receptors | ||
|---|---|---|
Expression pattern: nuclear | ||
Main diagnostic use | Expression in other tumors | Expression in normal cells |
Prostatic carcinoma | Osteosarcoma, apocrine breast carcinoma, Paget’s disease, salivary duct carcinoma, sebaceous carcinoma, basal cell carcinoma, mesonephric adenocarcinoma | Prostatic cells, Sertoli cells, Leydig cells, apocrine and sebaceous glands, skin, oral mucosa, hepatocytes |
Positive control: prostatic tissue | ||
Diagnostic Approach
Androgen receptor (AR) is a member of the steroid family of ligand-dependent transcription factors. Androgen receptor is expressed in different tissue types including prostatic glands and skin adnexa. Neoplastic prostatic glands are usually positive for AR, but studies show no direct correlation between the intensity of AR expression and the response to hormonal therapy [3]. The nuclear expression pattern of AR makes it useful for the immunohistochemical double stain with other antibodies with cytoplasmic or membranous expression pattern.
Diagnostic Pitfalls
The expression of AR is not restricted to prostatic carcinoma and can be found in other carcinoma types with similar morphology such as salivary duct carcinoma, breast carcinoma, and apocrine carcinoma.
Alpha-methylacyl-CoA racemase (AMACR, p504S) | ||
|---|---|---|
Expression pattern: cytoplasmic | ||
Main diagnostic use | Expression in other tumors | Expression in normal cells |
Prostatic adenocarcinoma, high-grade PIN | Gastrointestinal adenocarcinoma, hepatocellular and papillary renal cell carcinoma, carcinoma of the breast and ovaries, endometrial clear cell carcinoma, urothelial carcinoma, extramammary Paget’s disease, mesothelioma, lymphoma, pancreatic islet tumor | Periurethral glands, liver, salivary glands, sebaceous glands, renal tubular epithelium, pancreas epithelium, mesothelial cells |
Positive control: prostatic carcinoma | ||
Diagnostic Approach
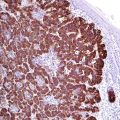
Alpha-methylacyl-CoA racemase (also known as p504S) is a member of the isomerases enzyme family involved in the metabolism of branched-chain fatty acids and synthesis of bile acids. It is expressed in the mitochondria and peroxisomes of various normal and neoplastic cells. p504S is overexpressed in prostatic carcinoma compared to benign prostatic glands (Fig. 13.1) [4, 5]. In combination with p63, alpha-methylacyl-CoA racemase (AMACR) is now widely used for the diagnosis of prostatic carcinoma (so-called PIN cocktail). p63 is a myoepithelial marker exhibiting a nuclear stain [6]. The immunohistochemical double stain with the PIN cocktail can show one of the following three results:
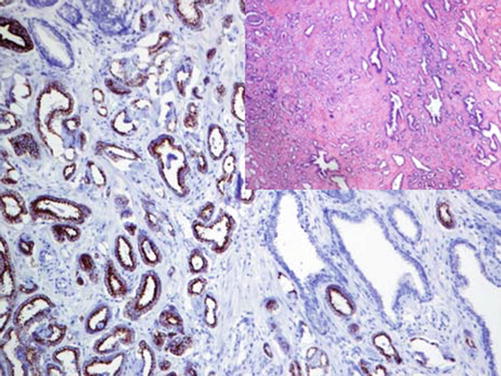
AMACR-positive prostatic glands lacking the p63-positive myoepithelial cells; a combination characteristic of neoplastic glands
AMACR-positive glands surrounded by p63-positive myoepithelial cells; characteristic of prostatic glands with high-grade PIN
AMACR-negative prostatic glands surrounded by p63-positive myoepithelial cells; characteristic of normal prostatic glands

Fig. 13.1
AMACR expression in luminal cells of prostatic adenocarcinoma
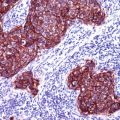
Low molecular weight cytokeratins such as CK5/6/14 can be used as alternatives to p63 in a separate reaction (Fig. 13.2).
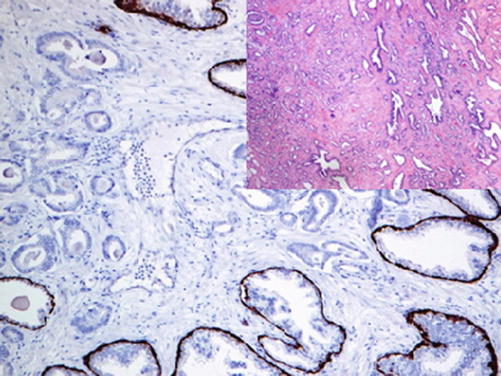

Fig. 13.2
Neoplastic glands of prostatic adenocarcinoma lacking the myoepithelial cells positive for high molecular weight cytokeratin (CK5/14)
Diagnostic Pitfalls
The expression of AMACR is found in many neoplasms types and cannot be considered as a specific marker of prostatic tumors [7].
NKX3.1 | ||
|---|---|---|
Expression pattern: nuclear | ||
Main diagnostic use | Expression in other tumors | Expression in normal cells |
Prostatic adenocarcinoma | GCNIS, breast carcinomas, subset of T-ALL | Prostatic tissue, salivary glands, mucinous bronchial glands |
Positive control: prostatic tissue | ||
Diagnostic Approach
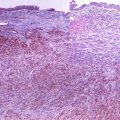
Is an androgen-regulated tumor suppressor gene located on chromosome 8p221.1 and strongly expressed in the nuclei of normal prostatic epithelium and persist in prostatic acinar carcinomas. NKX3.1 is a specific marker for primary prostatic carcinoma, and the intensity of the nuclear expression correlates with the differentiation grade of prostatic carcinoma, which can be very weak in poorly differentiated carcinoma (Fig. 13.3) [8].


Fig. 13.3
Metastatic prostatic carcinoma with strong nuclear NKX3.1 expression
Diagnostic Pitfalls
NKX3.1 is also expressed in testicular germ cells and seminoma in situ (GCNIS) but lost in invasive seminoma and embryonal carcinoma. Different expression intensity of NKX3.1 is also found in estrogen- and androgen-positive breast carcinomas, i.e., invasive lobular carcinoma [9]. Mucinous units of salivary and bronchial glands also reveal a nuclear expression of NKX3.1 which to consider in the interpretation of small biopsies [10]. Furthermore, the TAL-1 genetic aberration associated with a subset of T-ALL causes the activation of NKX3.1 expression in neoplastic lymphocytes [11].
ERG | ||
|---|---|---|
Expression pattern: nuclear | ||
Main diagnostic use | Expression in other tumors | Expression in normal cells |
Prostatic adenocarcinoma, endothelial tumors/angiosarcoma | Acute myeloid leukemia, solitary fibrous tumor, epithelioid sarcoma, meningioma | Endothelial cells |
Positive control: blood vessels | ||
Diagnostic Approach
V-ETS avian erythroblastosis virus E26 oncogene homolog (ERG ) encoded by the gene located on chromosome 21q22.3. ERG is a member of the ETS family transcription factors, which also include Fli-1 and EST-1. ERG is normally expressed in endothelial cells and tumors derived from these cells [12].
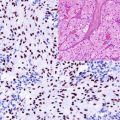
The ERG gene is the fusion partner of the TMPRSS2 gene involved in the regulation of androgen response. This genetic mutation is the most frequent genetic abnormality associated with prostatic carcinoma and found in 40–80% of the cases. This mutation generates the TMPRSS2-ERG gene fusion causing the overexpression of the ERG protein detected by immunohistochemistry (Fig. 13.4) [13, 14].
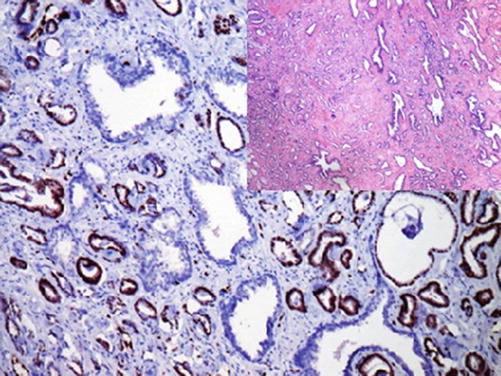

Fig. 13.4
Nuclear ERG expression in neoplastic cells of prostatic adenocarcinoma
The aberrant expression of ERG is also characteristic for the solitary fibrous tumor because of other genetic anomalies associated with this tumor. ERG expression is also reported in few other mesenchymal tumors including epithelioid sarcoma and fibrous meningioma [15, 16].
Despite this obvious lack of sensitivity, ERG positivity in a metastasis of unknown epithelial primary can be considered confirmative of prostate cancer.
Diagnostic Pitfalls
The immunohistochemical results using this marker must be carefully interpreted as positive staining is observed in about 29% of high-grade PIN and occasionally benign glands; thus, the gold standard remains the labeling of the myoepithelial basal cells [17]. Both antibodies to ERG and p63 can be used as a cocktail for the diagnosis of prostatic carcinoma but has less sensitivity than the above disrobed PIN cocktail.
Phosphatase and Tensin Homolog (PTEN):
PTEN is a tumor suppressor mentioned in a previous chapter. The loss of PTEN is found in a fraction of high Gleason prostatic carcinoma, which is usually resistant to the antiandrogen therapy. Furthermore, the loss PTEN expression is a useful marker to distinguish intraductal carcinoma from PIN usually positive for PTEN.
Immunoprofile of prostatic and seminal vesicle tumors | ||||
|---|---|---|---|---|
Tumor type | + in >90% (+) | + in 50–90% (+/−) | + in 10–50% (−/+) | + in <10% (−) |
Sclerosing adenosis of the prostate : | Preserved myoepithelial basal cells positive for high molecular weight cytokeratins (CK5/6/14, CK-34E12), p63, p40, LP34 | |||
Adenocarcinoma of the prostate: – Acinar adenocarcinoma – Ductal adenocarcinoma | CK8, CK18, CK19, PSA, PAP, NKX3.1, prostein, hK2, p504S (racemase) Diagnostic is the loss of basal myoepithelial cell layer: negativity for high molecular weight cytokeratins (CK5/6/14, CK-34E12), p63, p40 | Androgen receptors, ERG | CK7, | CK5, CK10, CK20, CEA, uroplakin |
Basal cell carcinoma of the prostate: | CK8, CK18, CK5/6/14, p63, p40, HER-2, androgen receptors, bcl-2 CK7 in luminal cells | P504S (AMACR), CK 20, PSA | ||
Neuroendocrine tumors: – Adenocarcinoma with neuroendocrine differentiation – Well-differentiated neuroendocrine tumor – Small and large cell neuroendocrine carcinoma | See neuroendocrine tumors | |||
Stromal tumor of uncertain malignant potential/stromal sarcoma: | CD34, PgR | ER | ||
Adenocarcinoma of seminal vesicle: | CK8, CK18, CK19, PAX-8, CA-125, CEA
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

| |||