(1)
Institut Bergonié, Bordeaux, France
Abstract
The MAP kinase pathway (or, better, the MAP kinase pathways) is certainly one of the main proliferation pathways, the best known and the one whose oncogenic alterations have elicited the highest number of studies devoted to these alterations. This pathway is activated downstream numerous membrane receptors, especially those of the EGF receptor family, and leads ultimately to the activation of transcription factors called MAPs (mitogen-activated proteins), which govern the transcription of numerous genes required for DNA replication and cell division. Besides the classical pathway, the best known, which will be presented in detail, several parallel MAP kinase pathways have been identified. They are activated by other stimuli and lead to the activation of other transcription factors involved in stress response, inflammation and development, rather than in cell proliferation.
In the previous chapter, we showed that the phosphotyrosine residues of the activated receptor could be recognised by a large number of adapter proteins bearing an SH2 or a PTB domain. We will start this chapter with some of these proteins, which associate receptor activation to a GTP exchange protein, which in turn activates a small G-protein of crucial importance, the RAS protein. RAS activation triggers a cascade of phosphorylations leading to the activation of transcription factors.
2.1 From Receptor Activation to RAS Activation
The growth factor receptor-bound protein 2 (GRB2) is an adapter protein with SH2 and SH3 domains. Its SH2 domain recognises specific phosphotyrosine residues of activated TKRs (Chap. 1). This allows the enrolment at the membrane of a GDP–GTP exchange factor (GEF) called SOS1 (son of sevenless homologue 1), which replaces GDP by GTP as a ligand of the membrane protein RAS. The RAS proteins belong to the large protein family of small G-proteins, and GDP–GTP exchange on a small G-protein is commonly mediated by GEFs. Whereas the pathway EGFR–GRB2–SOS1–RAS is the best studied, there exist multiple other pathway downstream activations of tyrosine kinase receptors: other SH2 domain-containing adapter proteins are able to bind phosphotyrosine residues of tyrosine kinase receptors. This is especially the case for FGF receptors, for which a protein called FRS (FGF receptor substrate) is phosphorylated by the receptor and then recognised, at the level of its phosphotyrosine residues, by GRB2 or another adapter protein.
Three RAS protein homologues play similar roles in various tissues: KRAS (Kirsten RAS), HRAS (Harvey RAS) and NRAS (neuroblastoma RAS). All small G-proteins function similarly: they display high affinity for GDP, which is replaced by GTP via a GEF. This exchange induces a change of conformation, triggering downstream activity (for instance, RAS activation by GTP leads in particular to its binding to a serine/threonine kinase called RAF). Small G-proteins possess an intrinsic GTPase activity, allowing the retroconversion of GTP to GDP by elimination of the GTP gamma phosphate group. This GTPase activity may be stimulated by a GTPase-activating protein (GAP), which constitutes therefore the third member of the functional triad GEF–small G-protein–GAP, which is involved in many cellular events outside the control of proliferation studied in this chapter.
There are other possibilities of RAS activation. In the pathway opened by TKR activation, the GEF is SOS1 and the GAP is RASGAP (p120RASGAP, gene RASA1). Another GEF for RAS is RASGRP (RAS guanyl nucleotide-releasing protein), which can be activated by other molecules: inositol trisphosphate (IP3) through Ca2+ mobilisation and diacylglycerol (DAG). These two molecules can be formed from phosphatidylinositol by phospholipase C gamma (PLCγ), which possesses a SH2 domain allowing its activation by a TKR (see Chap. 1). IP3 and DAG are ‘second messengers’ that can also be formed by phospholipase C beta (PLCβ), which is activated via another signalling pathway (Chap. 6) involving receptors with seven transmembrane domains called GPCR (large heterotrimeric G-protein-coupled receptors).
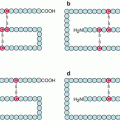
The RAS proteins are anchored to the plasma membrane through covalent bonds with a branched hydrophobic moiety constituted of 15 carbon atoms (farnesyl) or 20 carbon atoms (geranylgeranyl). These prenyl moieties, which are intermediates of cholesterol biosynthesis, are added as post-translational modifications at the level of the endoplasmic reticulum. Their binding to the RAS proteins occurs on a cysteine residue of the polypeptidic chain, borne by a –CAAX motif (C for cysteine, A for aliphatic amino acid (Val, Leu or Ile), X for Met or Ser), the three amino acids AAX being eliminated after prenylation by RAS-converting endopeptidase (gene RCE1) (Fig. 2.1). In addition, some RAS proteins undergo palmitoylation in the Golgi apparatus. This modification is a covalent binding of palmitic acid, a fatty chain of 16 carbon atoms, at the level of a cysteine residue located upstream the one that is prenylated. It is generally admitted that the recruitment of SOS1 to the membrane, thanks to its affinity for GRB2, enables its interaction with its RAS protein substrate and, as a consequence, RAS activation. This membrane anchoring is required for RAS activity.
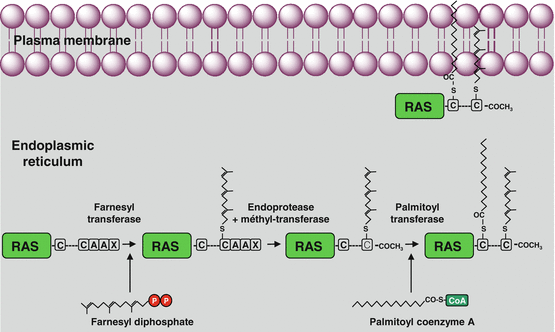

Fig. 2.1
Post-translational modifications of RAS proteins. The RAS proteins undergo, in the endoplasmic reticulum, covalent binding to a prenyl (farnesyl or geranylgeranyl) moiety on a cysteine residue included in a –CAAX box. The amino acids AAX are then deleted and replaced by an acetyl moiety at the C-terminal end. Later, a palmitic acid is bound to RAS proteins on another cysteine residue located some amino acids upstream of the prenylated cysteine. These modifications are required for the plasma membrane insertion of RAS proteins
When RAS becomes bound to GTP, it undergoes a conformational modification that allows the recruitment to the membrane of a serine/threonine kinase which is a RAF protein in the MAP kinase pathway. Besides being the main activator of the MAP kinase pathway described below, RAS has many effector proteins (Fig. 2.2): RAS can directly activate the catalytic subunit of PI3 kinase (Chap. 3), as well as other enzymes such as phospholipase C epsilon (PLCε). It can also regulate the activity status of other small G-proteins by activating their GEFs or GAPs: this is the case for RAL, which plays a role in endocytosis, and for RHO family G-proteins, which are involved in the control of cytoskeleton movements. The RAS proteins are, therefore, at an important crossroad of signalling pathways. RAS protein activation is a rapidly reversible, since intrinsic RAS GTPase activity, stimulated by the RASGAP protein, rapidly deactivates RAS by leading it back to its inactive form, RAS-GDP, which is unable to recognise RAF and attract it to the plasma membrane. RASGAP presents an SH2 domain allowing it to be recruited at the membrane level by a TKR to exert its action. The successive steps of the MAP kinase pathway are described below (Fig. 2.3).
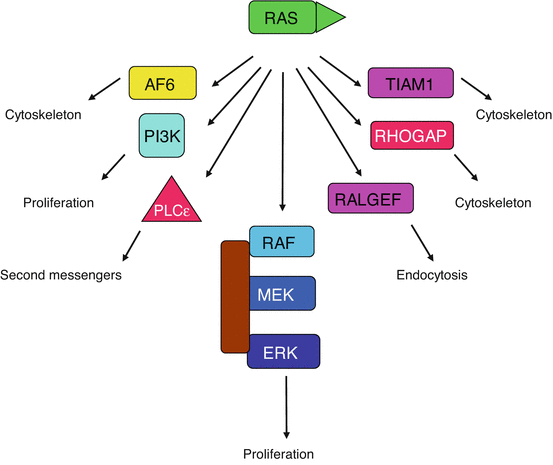
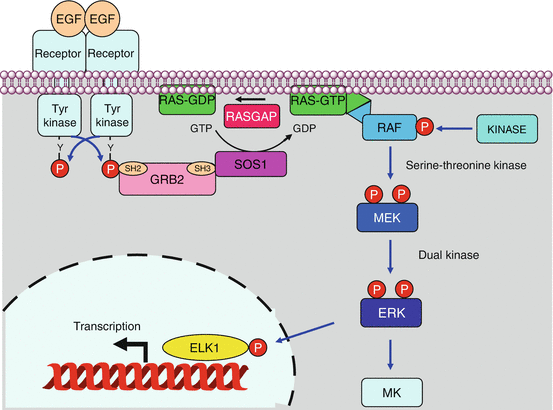

Fig. 2.2
Effectors of the RAS proteins. The RAS proteins have a multiplicity of targets and can activate a large variety of signalling pathways. The MAP kinase pathway is central in this respect, but activation of phospholipase Cε (PLCε) and of PI3 kinase also concurs to proliferation, while the activation of G-protein exchange factors (GEFs) and GTPase-activating proteins (GAPs) entails several roles on cell processes such as endocytosis and cytoskeleton organisation

Fig. 2.3
MAP kinase pathways. In the canonical signalling pathway, the activation of a TKR enables the recruitment of an SH2 domain-containing protein, GRB2; the SH3 domain of GRB2 is recognised by the SOS1 protein, a GDP–GTP exchange factor for RAS proteins. RAS–GTP can recruit to the plasma membrane a RAF protein, which thus can be phosphorylated by a variety of other kinases (PKA, PAK, SRC) activating its kinase function. RAS–GTP is then deactivated by its own GTPase activity stimulated by a RASGAP (RAS GTPase-activating protein) protein. RAF phosphorylates and activates a MEK protein, which in turn activates an ERK protein, which in turn phosphorylates and activates a transcription factor such as ELK1 or a kinase of the MK family
2.2 Kinase Cascade
The direct target of RAS in the MAP kinase pathway is a serine/threonine kinase called RAF. There are three distinct RAF proteins, ARAF, BRAF and CRAF (gene RAF1). RAF binding to RAS–GTP induces a conformational change which unveils an activation loop allowing their phosphorylation and consequently their activation. The activation process of the RAF proteins is complex and not completely deciphered; it involves RAF dimerisation and phosphorylation on various sites by several kinases such as cyclic AMP-activated protein kinase A (PKA), which is brought into play following GPCR activation (Chap. 6); the cytoplasmic tyrosine kinase SRC, activated by several types of stimulus; the JAK kinase activated by cytokine receptors (Chap. 4) or the kinase named PAK1 (p21-activated kinase 1), which operates after activation of the integrin (Chap. 10) or semaphorin (Chap. 11) pathways.
RAF proteins may undergo phosphorylation on several distinct sites. Two of them are in the N-terminal domain, on an SSYY motif where the first serine residue and the second tyrosine residue must be phosphorylated; however, for BRAF, the first serine residue is constitutively phosphorylated, and the two tyrosine residues are replaced by aspartic acid residues whose negative charge is constitutively ‘phosphomimetic’. Two other phosphorylation sites are located on a threonine and a serine residue (Thr599 and Ser602 for BRAF). Whereas ARAF and CRAF require four phosphorylated residues for activity, BRAF requires only two; the consequences upon RAF-mediated oncogenesis will be detailed below.
Downstream RAF kinases are the kinases MEK1 and MEK2, and downstream MEK1/2 are the kinases ERK1 and ERK2 (extracellular signal-regulated kinases). This constitutes, therefore, a three-step kinase system, the RAF–MEK–ERK cascade. The RAF kinases are also called MAP3 kinases (MAP3K or MAPKKK), the MEK kinases are MAP2K or MAPKK and the ERK kinases are the proper MAP kinases (MAPK). It will be shown later that this RAF–MEK–ERK ‘module’ is only one among others and that other parallel MAP kinase pathways exist, with similar three levels of phosphorylation. They are acting in stress response or inflammation rather than in cell proliferation. A total of 25 MAP3K, 7 MAP2K and 13 MAPK have been identified in the superfamily of serine/threonine kinases. The official gene names of the MEK and ERK proteins studied here differ from their common denomination: MAP2K1 and MAP2K2 for MEK1 and MEK2, MAPK3 and MAPK1 for ERK1 and ERK2, respectively.
MEK1/2 activation is obtained by phosphorylation of two close serine residues: Ser218 and Ser222 for MEK1 and Ser222 and Ser226 for MEK2, located in the activation loop of the catalytic centre. The most active kinase on MEK1/2 is BRAF, followed by CRAF, while ARAF has lower activity. MEK1/2 is in turn able to phosphorylate ERK1/2. MEKs are dual kinases, able to phosphorylate both a threonine and a tyrosine residue, also at the level of the activation loop, following each other as TEY (Thr202–Glu203–Tyr204 for ERK1 and Thr185–Glu186–Tyr187 for ERK2). Kinase specificity to the kinase of lower level depends on interactions between protein–protein binding domains rather than on different catalytic activities.
The three successive steps of kinase action are not scattered in the cytoplasm. Scaffold proteins are able to maintain the three sequentially acting kinases as a functional complex. For the RAS–RAF–MEK–ERK pathway, this scaffold protein is KSR1 (kinase suppressor of RAS-1) and operates at the level of the plasma membrane (Fig. 2.4). Other scaffold proteins operate for this MAP kinase module at other sites: endosomes, Golgi apparatus and focal adhesions; the scaffold protein of the Golgi apparatus is recruited by the exchange factor RASGRP1, activated by the second messengers generated by the PLCs γ and β, as mentioned earlier.
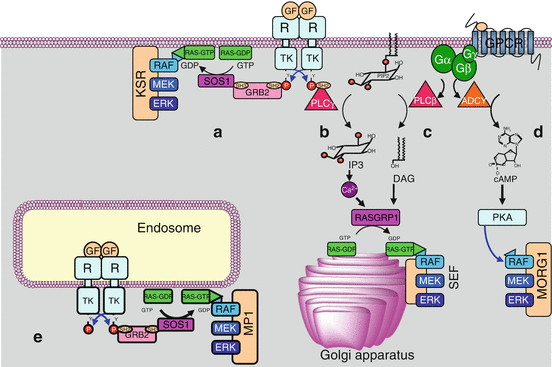

Fig. 2.4
Kinases organisation and scaffold proteins. The three MAP kinases acting sequentially are not scattered in the cytoplasm but assembled by scaffold proteins. (a) The signals brought by a growth factor (GF) to a tyrosine kinase receptor (TKR) activate the RAS–RAF–MEK–ERK pathway at the plasma membrane level, thanks to the scaffold protein KSR (kinase suppressor or RAS). (b) They can also activate phospholipase C gamma (PLCγ) leading, through the second messengers inositol trisphosphate (IP3) and diacylglycerol (DAG), to the activation of the RAF–MEK–ERK cascade in the Golgi apparatus, thanks to the scaffold protein SEF (similar expression to FGF gene). (c, d) Signals activating a G-protein-coupled receptor (GPCR) can activate the RAF–MEK–ERK pathway, either by generating the second messengers IP3 and DAG produced by PLCβ (c), or by generating the second messenger cAMP (cyclic AMP), which activates the protein kinases A (PKA), using the scaffold protein MORG1 (MAPK organiser 1) (d). (e) The activation of a TKR at the endosomal level leads to a classical activation of a RAS protein, then to the sequence RAF–MEK–ERK using the scaffold protein MP1. Other systems may exist, involving the same kinase cascade RAF–MEK–ERK and other scaffold proteins such as β-arrestin or paxillin
Downstream ERK1 and 2 are two types of substrates: a large set of transcription factors involved in proliferation and various kinases of the MK (MAPK-activated protein kinases) family; they are presented in paragraph 4. Negative regulators of the MAP kinase pathway consist in a series of dual phosphatases, called MKP (MAP kinase phosphatases) or DUSP (dual specificity phosphatases). They operate at the level of phosphothreonine and phosphotyrosine residues, with variable substrate specificity, at the level of the activating sequences TXY.
2.3 Other Signalling Modules
As mentioned earlier, there are several signalling modules operating downstream RAS proteins, organised as phosphorylation cascades, with distinct MAP3Ks, MAP2Ks, MAPKs and scaffold proteins. These are the JNK module, the p38 module and the ERK5 module. Figure 2.5 presents these pathways, which are parallel to the RAF–MEK–ERK canonical pathway. It is important to note that their cellular effects involve differentiation or apoptosis rather than cell proliferation, exemplifying the difficulty to assign a unique function to a given signalling pathway: the ‘cellular context’, i.e. the conditions in which these effects are exerted, plays a crucial role.
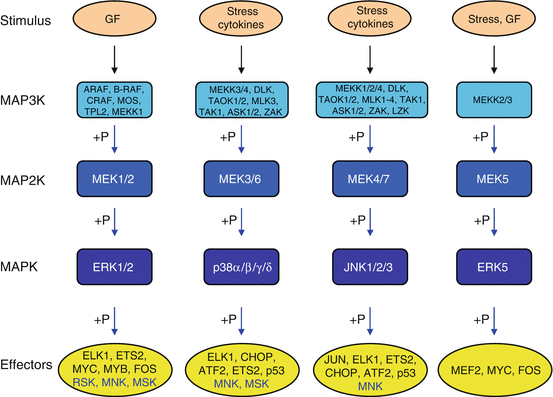

Fig. 2.5
The different modules of kinase cascades. Five main modules of multistep kinases have been identified; all of them originate from a stimulus (growth factor, cytokine, stress, etc.) which activates a MAP3K, which phosphorylates and activates a MAP2K, which phosphorylates and activates a MAPK, which phosphorylates and activates an effector (transcription factor or another serine/threonine kinase). The initial stimulus may originate from a small G-protein such as RAS or from phosphorylation by a fourth-level kinase (MAP4K). From left to right, the classical ERK1/2 pathway, the p38 pathway, the JNK pathway and the ERK5 pathway
There are three JUN N-terminal kinases: JNK1, 2 and 3 (genes MAPK8, 9 and 10), also known as SAPKs (stress-activated protein kinases). They are activated in response to various stresses such as heat shock, ionising radiations or DNA damage rather than by growth factors. TNF (tumour necrosis factor) and its analogues as well as the WNT ligands (Chap. 7) also activate this pathway. They are phosphorylated on a TPY sequence (Thr183–Pro184–Tyr185 for JNK1) by MEK4 and MEK7 (genes MAP2K4 and MAP2K7), which are themselves phosphorylated by diverse MAP3K at positions Ser257 and Thr261 for MEK4 and Ser271 and Thr275 for MEK7 (see Fig. 2.5). The JUN kinases phosphorylate transcription factors such as JUN, MYC and ELK1, which in turn regulate the expression of specific genes involved in cell death or survival, differentiation or proliferation. They also phosphorylate several kinases of the MK family.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree