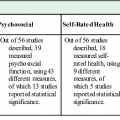
Steve Iliffe The aging population in industrialized societies is a challenge for primary care. Older people with multimorbidity, disabilities, cognitive impairment, and frailty are not well served by brief encounters with practitioners whose working style is reactive rather than proactive. In a competition for attention and clinical assessment, the complex patient is at a disadvantage compared with the younger, more articulate patient with a single problem to solve. The clinical task is made more difficult because population aging is a diverse and complex process, with older individuals moving along a spectrum of fitness to frailty at different speeds. Identifying those individuals most likely to benefit (and least likely to experience harm) from interventions to delay their progress along this spectrum is becoming a priority for primary care practitioners,1 but it is not clear how to do it. The evidence base for interventions to limit or even reverse frailty and pre-frailty is shallow. The overarching task is to overcome the obsolescence of the current health and social care systems, but there is a pressing need for primary care practitioners to acquire the skills needed to manage clinical complexity and frailty. This chapter describes how primary care needs to reconfigure itself to manage an increasingly frail population, focusing on promoting healthy aging; case finding, case management, and interventions for frailty; understanding patients’ perspectives on frailty; working with care home residents; and providing end-of-life care. Frailty as a state of heightened and disproportionate vulnerability resulting from multisystem failure and is distinct from multimorbidity and disability.2 One quarter to one half of people older than 85 years is frail, and these people have significantly increased risk of disability, hospitalization, long-term care, and death.3 Frailty, however defined, is more common in women and some ethnic minorities, increases in prevalence with age, and is associated with poor survival.4 However, up to three quarters of people older than 85 years might not be frail; frailty is not synonymous with being among the oldest old.2 It is a dynamic process that evolves over time, offering opportunities for prevention and remediation.5 The dependency oscillations observed in older people who are frail reflect the often marked changes in functional ability seen in clinical practice. However, at present, progression of frailty is more common than improvement, and the onset of frailty frequently results in a spiral of decline.6 Frailty also has psychological and social dimensions. It is associated with worse well-being, taking into account depression and functional limitations, while financial resources may act as a partial buffer against the psychological effects of frailty.7 Maintaining a stronger sense of psychological well-being in later life may protect against the development of physical frailty, although the mechanisms underlying this are unclear.8 There appears to be a social gradient in frailty, with educational level, income adequacy, and income satisfaction being associated with frailty.9 Older adults who are poor and live in deprived neighborhoods are those most likely to develop frailty.10 Frailty provides a conceptual basis for moving away from organ- and disease-based medical approaches toward a more integrative model of health, and therefore it fits the biopsychosocial model of generalism very well.11 Its identification and management in general practice is, then, a test of the claims made for the biopsychosocial model. The concept of frailty, and measures based on it, may also provide a more user-centered approach to developing services that cuts across unidisciplinary preoccupations and definitions of clinical effectiveness. It also has potential in the identification of individuals requiring integrated care, in the planning of care packages, and in the monitoring of the health status of patients and service users. A caveat here: most frail older adults report that they receive sufficient help for their physical needs but not for their psychosocial needs.12 A wide variety of frailty measures are available, and further work is required to establish which are the most suitable for these different applications.13 In most people, multimorbidity, disability, and frailty develop slowly over decades and may be difficult to distinguish. For example, the duration of unhealthy behaviors in mid-life, particularly poor diet and physical inactivity, increases the likelihood of frailty features like slow walking speed and reduced grip strength emerging in later life,14 but these features will coexist with other consequences of inactivity and poor nutrition such as heart disease, obesity, and diabetes, among others. Aging well may therefore depend in part on changing behaviors and modifying risks for disability and frailty earlier in life. Unfortunately, refocusing attention “upstream” does not solve the problem because we do not yet know how to maximize the uptake of preventive activities in mid-life across the population. Prevention holds the promise of maintaining good health by testing, diagnosing, and treating conditions before they cause symptoms. However, prevention can harm as well as help when tests or treatments for asymptomatic conditions cause immediate complications. “Lag time to benefit” is defined as the time between a preventive intervention (when complications and harms are most likely) to the time when improved health outcomes are seen. Just as different interventions have different magnitudes of benefit, different preventive interventions have different lag times to benefit, ranging from 6 months for statin therapy for secondary prevention to more than 10 years for prostate cancer screening. Many standardized measures, such as relative risk, odds ratio, and absolute risk reduction, quantify the magnitude of benefit (“How much will it help?”). The measures and methodologies to calculate a lag time to benefit (“When will it help?”) are underdeveloped and often not reported.15 So, for example, the promotion of physical activity in community settings (which has been a disappointing experience, to date) may be resisted because the benefits, including the postponement of disability and frailty, are distant while the disadvantages are immediate. Interventions in later life are therefore more attractive because of the shorter time scale to demonstrate effectiveness and because of the proximity of benefit. In primary care such interventions take the form of health maintenance (e.g., through weight control, smoking cessation, and exercise promotion) and “anticipatory care,” a broader approach to case finding and case management of long-term conditions. Unfortunately, and despite substantial research efforts over decades, health maintenance and anticipatory care for older people in the community have not yet been shown to be clinically effective or cost-effective.16 Their history provides a salutary lesson for researchers as well as those developing services. Health promotion trials for older people in the United States, United Kingdom, and Denmark up to 199017 showed a rise in patient morale, increased referrals to all agencies, reduced duration of inpatient stay (sometimes), increased inpatient rates (mostly because of respite care), reduction in mortality (in some trials), but no improvement in functional ability and increased workload for general practitioners unless alternative services bypassed primary care. Subsequent studies were essentially negative, and only recently has research begun to show more promising outcomes. For example, the largest community-based study in the United Kingdom, the MRC 75+ assessment trial, showed little or no benefits to quality of life or health outcomes.18 Similarly, a systematic review of 15 trials of preventive home visits published in 2000 showed no clear evidence of effectiveness,19 and the ProAge study in 2006 showed no change in health risk behaviors in older people.20 There are now some grounds for thinking that interventions can have a positive impact. Comprehensive geriatric assessment (CGA) followed by tailored case management is beginning to show an impact on function.21 One clinical trial showed that health educators doing preventive home visits can improve older people’s functional mobility.22 Another study provided evidence that nurse-led case management impacts positively on functional ability, caregiver burden, and satisfaction.23 Given that preventive interventions have limited impact on the development of disability or frailty, primary care practitioners are left to manage the impact of these states on their older patients. Whereas disability may be evident, identifying frailty can be more difficult; practitioners may need help in recognizing and responding to it. The point about recognition is not that it is diagnostic of frailty but rather that it helps physicians identify patients who may benefit from more detailed assessment. Primary care would be well placed for the early identification and management of frailty in older adults if a simple, easy-to-use measure to identify frailty specifically in primary care settings were available.24–26 A recent review recommends a two-step approach to identifying individuals who would benefit from a further detailed assessment.27 However, there are a number of problems that complicate the task of recognition. The validity and diagnostic accuracy of instruments for frailty have not been tested in well-defined community-based longitudinal studies. Several of the frailty instruments are also derived from small-scale studies, including some that are cross-sectional and not primary care based. There is a need for a simple screening tool for frailty for use in general practice based on large prospective studies of community-dwelling older populations. Despite their scientific validity, the “Fried frailty phenotype” (capturing a syndrome) and the “frailty index” (capturing a state) are difficult to apply in routine clinical practice in primary care, because they rely on objective measures such as grip strength (in the frailty phenotype) or the number of different deficits (in the frailty index). Most studies have assessed screening tools for frailty that are not directly applicable for use by general practitioners as first-stage screening instruments, but recently developed instruments have been assessed for use in primary care; these include the SHARE (Survey of Health, Ageing and Retirement in Europe) frailty instrument, the Groningen Frailty Indicator, the Tilburg Frailty Indicator, and the Edmonton Frail Scale. These measures also are extensive (approximately 15 to 20 items) and/or include objective measures (such as the timed get-up-and-go test) and therefore may not be suitable as brief, first-stage screening tools; they may be more useful as second-stage assessment instruments. A single-item assessment would be an ideal first stage in frailty case finding. Gait speed is the most predictive single factor associated with frailty,2 whereas fatigue that restricts activity, although common, is often episodic and of short duration and is a less accurate predictor of frailty.28 The second stage of case finding could then include use of a validated instrument such as the Edmonton Frail Scale, a multidimensional assessment instrument that includes the timed get-up-and-go test and a test for cognitive impairment. It is quick to administer (less than 5 minutes) and is valid, reliable, and feasible for routine use by nongeriatricians.29 There are alternatives, and the properties of some are compared in Table 116-1.25 TABLE 116-1 Comparison of Four Frailty Scales
Managing Frailty
Roles for Primary Care
Introduction
What Is Frailty and Who Is Frail?
Promoting Healthy Aging
Case Finding Methods
Groningen Frailty Indicator (GFI)
Frailty Staging System
Frailty and Autonomy Scoring Instrument Leuven (FRAIL)
Edmonton Frail Scale
ADLs
✓
✓
✓
✓
IADLs
✓
✓
✓
✓
Activities outside
✓
✓
✓
✓
Sensory functions
✓
✓
✓
Medication
✓
✓
✓
Memory
✓
✓
✓
✓
Orientation
✓
✓
✓
Behavior
✓
✓
✓
Social contacts
✓
✓
Familial functioning
✓
✓
Ability to plan things
✓
Finances
✓
Feeling fit/health status
✓
✓
Weight loss
✓
✓
Continence
✓
✓
✓ ![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Managing Frailty
116