The advent of targeted agents for the treatment of advanced renal cell carcinoma has led to dramatic improvements in therapy. However, the chronic use of these medications has also led to the identification of new toxicities that require long-term management. Effective management of toxicity is needed to maximize the benefits of treatment and improve patients’ quality of life. In addition, toxicity from these agents may affect treatment compliance, particularly with daily oral agents. This review delineates the toxicities that require monitoring, the underlying pathophysiology (when known), and treatments that may have benefits in relieving symptoms and side effects.
The advent of targeted agents for the treatment of advanced renal cell carcinoma has led to dramatic improvements in therapy. However, the chronic use of these medications has also led to the identification of new toxicities that require long-term management. The vascular endothelial growth factor (VEGF) receptor inhibitors (sunitinib, sorafenib, and pazopanib), VEGF-ligand inhibitor (bevacizumab), and mTOR inhibitors (temsirolimus and everolimus) each present different challenges for patient management. Quality of life for patients on chronic therapy has become increasingly important, and effective management of toxicity is needed to maximize the benefits of treatment. In addition, toxicity from these agents may affect treatment compliance, particularly with daily oral agents.
Despite being “targeted” agents, these drugs affect multiple organ systems that have the potential to impair quality of life and function. Nearly every organ system is affected to some degree. This review delineates the toxicities that require monitoring, the underlying pathophysiology (when known), and treatments that may have benefits in relieving symptoms and side effects.
Cardiovascular toxicities
VEGF inhibition by the approved anti-VEGF therapies has differential effects on the VEGF-VEGF receptor axis in tumor cells and normal tissues. In noncancer tissues, endothelial dysfunction and microvascular rarefaction set the stage for the development of hypertension (HTN), cardiomyopathy, thrombotic microangiopathy, and proteinuria.
Hypertension: The Problem
The most relevant cardiovascular side effect in clinical practice is HTN. The recognition of this side effect is an important issue because poorly controlled HTN can lead to serious cardiovascular events. HTN is an established risk factor for coronary heart disease, stroke, heart failure, and end-stage renal disease. Anticipation of HTN complications with anti-VEGF therapies, early detection, and personalized management may improve clinical outcomes and tolerance.
HTN de novo or worsening control of a preexisting diagnosis after the introduction of antiangiogenic treatment may indicate underlying mechanisms such as renal thrombotic microangiopathy or glomerular lesions, but more commonly it is isolated HTN secondary to treatment itself. HTN induced by antiangiogenic drugs is probably related to an increase in systemic vascular resistance (SVR). It is thought that decreased production of nitric oxide (NO) in the wall of arterioles and other resistance vessels is one of the main involved mechanisms. VEGF increases NO synthesis through upregulation of endothelial NO synthase, and VEGF inhibition diminishes NO synthesis. Measurements of urinary nitrate levels suggest that they decline with anti-VEGF tyrosine kinase inhibitors (TKIs), supporting this hypothesis. In anticipation of cardiovascular complications with anti-VEGF therapies, early detection and personalized management may improve clinical outcomes and tolerance.
The incidence of hypertension of any grade was reported to range from 9% to 30% in a recent review of randomized controlled trials, but this incidence could be even higher if a more strict definition and classification of HTN were used such as the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC7), rather than the National Cancer Institute Common Terminology Criteria for Adverse Events. Three meta-analyses of phase 1, phase 2, and phase 4 trials conducted in various types of cancer found a respective incidence of high-grade hypertension of 8.3% (95% confidence interval [CI] 5.6–12.1) for sunitinib, 6.5% (95% CI 1.8–21.1) for sorafenib, and 7.1% (95% CI 3.4–14.1) for bevacizumab in the subgroup of metastatic renal cell carcinoma (mRCC) patients.
On the other hand, HTN induced by anti-VEGF agents may be a predictive factor of oncologic response. In a retrospective analysis of 544 mRCC patients treated with sunitinib, the 441 patients presenting high systolic blood pressure had a statistically significantly improved progression-free survival (PFS) (12.5 vs 2.5 months) and overall survival (OS) (30.5 vs 7.8 months) with respect to other patients. Similarly, the 362 patients presenting high diastolic blood pressure had a statistically significantly improved PFS (13.4 vs 5.3 months) and OS (32.1 vs 15.0 months) with respect to other patients. Similar observations are reported for axitinib. In fact, the importance of hypertension as a potential clinical biomarker is prospectively being explored in the dose titration trial of axitinib (NCT00835978).
Hypertension Management
The proper management of hypertension is of fundamental importance in mRCC patients, but it can only be extrapolated from guidelines available for the general population. Lifestyle modifications should be encouraged, but these nonpharmacologic strategies are not always suitable for patients with altered performance status related to metastatic cancer necessitating early drug intervention.
No clear recommendation for an antihypertensive agent can be made in this context because of a lack of controlled studies addressing the subject. Only one randomized study showed a beneficial effect of use of a calcium channel blocker (CCB) to prevent or minimize HTN secondary to antiangiogenic therapy. Nitrates seem as effective as well. Blood pressure (BP)-lowering drugs should be individualized to the patient’s clinical circumstances, and angiogenic inhibitors should be dose reduced and sometimes withheld in patients who have experienced hypertensive crisis. Treatment suspension is mandatory in the case of life-threatening events.
The medical literature gives no indication that cancer-affected patients with HTN or pre-HTN should be managed in a way other than that recommended in the JNC7 guidelines. For patients with stage 1 or 2 uncomplicated HTN, the target BP level is less than 140/90 mm Hg. Current guidelines from the National Kidney Foundation, which have not been specifically validated in patients with cancer-related kidney diseases, recommend a target BP of 125/75 mm Hg or less, as tolerated, for patients with diabetes mellitus, proteinuria, or reduced kidney function; alternatively, the recommended BP goal is 130/80 mm Hg. Indeed, in cancer patients with comorbidities such as chronic kidney disease, a target BP level of less than 135/85 mm Hg may be acceptable. BP monitoring while receiving angiogenic inhibitors should be undergone at least weekly for the first 8 weeks and before any infusion or cycle thereafter.
Antihypertensive agents should be selected according to patient’s comorbidities, drug interactions, and contraindications. Given the efficacy of angiotensin-converting enzyme (ACE) inhibitors and angiotensin-2 receptor antagonist (ARA), clinicians may consider using one as a first-line agent when a patient’s plasma creatinine is less than 2 mg/dL and there is no evidence of hyperkalemia or renal artery stenosis. ACE inhibitors or ARA may be preferred for those patients with proteinuria, chronic kidney disease risks, or metabolic syndrome. The nondihydropyridine CCBs, such as verapamil and diltiazem, are cytochrome P450 3A4 (CYP3A4) inhibitors, and nifedipine, a dihydropyridine CCB, has been shown to induce VEGF secretion. In the absence of available data from clinical studies, CCB dihydropyridines, such as amlodipine and felodipine, are the preferred class of CCB, and the nondihydropyridine CCB should be contraindicated or used cautiously in conjunction with angiogenic inhibitors metabolized by CYP3A4. Dihydropyridine CCB should be preferred in elderly or black patients. A general guideline on the use of antihypertensive agents is shown in Table 1 .
| Antihypertensive Class | Interaction |
|---|---|
| ACE, ARB, diuretics, β-blockers, α-blockers, nitrate derivatives | Minor degree of interaction |
| Nifedipine, calcium channel blockers | Some degree of interaction |
| Verapamil and diltiazem | Not recommended for use with oral drugs using CYP3A4 pathway (only with bevacizumab) |
Arterial thromboembolic events
VEGF-directed agents can alter the hemostatic balance by interfering with the integrity of the endothelial cells, decreasing the production of nitric oxide, and altering membrane lipids. These agents have been associated with both coagulative and bleeding disorders. Meta-analyses have shown that bevacizumab was significantly associated with an increased risk of arterial thromboembolism (relative risk [RR], 2.08 (95% CI 1.28–3.40, P = .003)). A subsequent report confirmed an overall RR for arterial thromboembolic events (ATEs) with bevacizumab-based therapy versus controls of 1.46 (95% CI 1.11–1.93, P = .007) as well as an increased risk of venous thromboembolism (RR for all grades, 3.0; 95% CI, 1.23–7.33; RR for grade 3–4 events, 2.86; 95% CI, 0.62–13.24).
Similarly, treatment with the VEGF receptor TKIs sunitinib and sorafenib is associated with a significant increase in the risk of ATEs. The RR of ATEs associated with sorafenib and sunitinib was 3.03 (95% CI, 1.25–7.37; P = .015) compared with control patients with an overall incidence of 1.4%. This risk did not depend on the type of TKI used or type of malignancy (renal cell carcinoma [RCC] vs non-RCC).
Arterial thromboembolic events
VEGF-directed agents can alter the hemostatic balance by interfering with the integrity of the endothelial cells, decreasing the production of nitric oxide, and altering membrane lipids. These agents have been associated with both coagulative and bleeding disorders. Meta-analyses have shown that bevacizumab was significantly associated with an increased risk of arterial thromboembolism (relative risk [RR], 2.08 (95% CI 1.28–3.40, P = .003)). A subsequent report confirmed an overall RR for arterial thromboembolic events (ATEs) with bevacizumab-based therapy versus controls of 1.46 (95% CI 1.11–1.93, P = .007) as well as an increased risk of venous thromboembolism (RR for all grades, 3.0; 95% CI, 1.23–7.33; RR for grade 3–4 events, 2.86; 95% CI, 0.62–13.24).
Similarly, treatment with the VEGF receptor TKIs sunitinib and sorafenib is associated with a significant increase in the risk of ATEs. The RR of ATEs associated with sorafenib and sunitinib was 3.03 (95% CI, 1.25–7.37; P = .015) compared with control patients with an overall incidence of 1.4%. This risk did not depend on the type of TKI used or type of malignancy (renal cell carcinoma [RCC] vs non-RCC).
Cardiotoxicity
Cardiac damage from TKI treatment may be a largely underestimated phenomenon. VEGF may play a critical role in coordinated tissue growth and angiogenesis in the heart. Blocking this pathway may lead to a disruption in cardiac remodeling and consequently induce heart failure. Careful cardiovascular monitoring as well as prophylactic cardiovascular treatment is essential, and may allow continuation of aggressive therapy for the underlying cancer.
In the phase 2 clinical trials of sunitinib in RCC, 8.9% of patients developed a reduction in left ventricular ejection fraction (LVEF). The incidence of left ventricular cardiac dysfunction (LVCD) reported with sunitinib in the phase 3 trial was 13%, with 3% of patients experiencing grade 3 events, but the incidence was not different between the sunitinib and interferon groups. Interferon, however, may cause cardiomyopathy as well. The sunitinib expanded access trial reported cardiac failure of any grade in less than 1% of patients, although a baseline cardiac evaluation was not performed per protocol.
In a retrospective analysis, a decline in cardiac function was noted in 3% of patients treated with sunitinib. Heart failure was preceded by hypertension in all patients, and the resultant left ventricular dysfunction was not completely reversible, even on discontinuation of sunitinib. A single-center analysis of patients receiving sunitinib for renal cell cancer or gastrointestinal stromal tumor described 15% with symptomatic congestive heart failure (CHF); importantly, in this report cardiac function appeared to improve with discontinuation of sunitinib and introduction of CHF medication. There is no general consensus on the frequency required for the LVEF monitoring. Patients presenting a history of coronary artery disease and/or hypertension can have their ejection fraction monitored every 2 or 3 cycles. Treatment should be suspended for grade 3 to 4 LVCD and the patient referred to a specialist.
Serious cardiotoxicity was infrequent in the prospective trials of sorafenib. In one phase 3 trial, the rates of cardiac ischemia/myocardial infarction were not statistically different in the sorafenib and placebo arms. In another phase 3 study, the incidences of cardiac ischemia and infarction were significantly higher in the sorafenib arm (RCC: 3% vs 1%; P <.01). An independent review of two studies by the Food and Drug Administration indicated that the incidence of ischemia/infarction was higher in the sorafenib group (2.9%) than in the placebo group (0.4%). A prospective evaluation of 74 patients with renal cell cancer receiving sorafenib or sunitinib with detailed cardiovascular monitoring observed up to a 34% incidence of cardiac toxicity; half were symptomatic, with significant decreases in LVEF in 12% of patients. Other important cardiac toxicities (eg, arrhythmias, electrocardiographic [ECG] changes, cardiac enzymes elevations, acute coronary artery syndrome) were also seen, with ECG changes in 16% of cases.
CHF has been occasionally reported for bevacizumab. A meta-analysis assessing the risk of serious CHF in patients with breast cancer receiving bevacizumab revealed an overall incidence of 1.6% with an RR of CHF in bevacizumab-treated patients of 4.74 (95% CI 1.66–11.18; P <.001) compared with placebo.
Experience with the VEGF receptor antagonists suggests related cardiac toxicities may be reversible on withdrawal of those agents and/or initiation of cardiovascular treatment, and that the VEGF receptor TKIs can be safely resumed, sometimes with a lower dose. It is not known if this treatment paradigm applies to bevacizumab-treated patients, as the pharmacokinetics of bevacizumab are different and the half-life (approximately 21 days) is prolonged.
Better cooperation among clinical and translational cardiologists and oncologists will be required from the early phases of drug development and for daily management of patients. It will be essential to identify effective strategies to address the cardiovascular on-target toxicity. Prospective studies specifically conducted in mRCC patients treated with targeted agents (eg, VEGF receptor TKIs) are presently lacking and are urgently needed.
Bleeding
A meta-analysis has shown that bevacizumab was significantly associated with an increased risk of bleeding (RR for high-grade events, 3.7; 95% CI 2.6–5.5) in mRCC patients, compared with controls. Sunitinib and sorafenib are also associated with an increased risk of bleeding (RR for all grades, 2·74; 95% CI 1·32–5·69; RR for high grade, 5.14; 95% CI 1.35–19.64) in patients with mRCC. A higher incidence of intracerebral hemorrhage has been recently reported in patients with metastatic RCC treated with TKIs targeting the VEGF receptors sorafenib and sunitinib. This retrospective study showed an incidence of 7% (5 of 67) of fatal intracerebral hemorrhage, with 4 out of 5 patients presenting with brain metastasis. This result was probably related to uncontrolled HTN at diagnosis.
This increased risk should be managed with a thorough medical history, frequent clinical examinations, and thorough investigation of suspicious symptoms. Grade 2 to 4 thrombotic or bleeding events require treatment suspension and appropriate treatment until recovery to grade 1.
Metabolic effects of targeted therapies
Use of mTOR inhibitors is associated with alteration in glucose and lipid homeostasis, reflective of the central role of the mTOR protein in these processes. TKIs have been implicated in thyroid dysfunction.
Hyperglycemia
Grade 3 to 4 hyperglycemia occurs in up to 15% of patients treated with mTOR inhibitors. In patients with diabetes mellitus treated with these agents, frequent monitoring of blood sugar and adjustment of medications is necessary to prevent hyperglycemic crises. For patients without diabetes mellitus, frequent screening of blood sugar is required to monitor for the emergence of hyperglycemia. Because patients with RCC frequently have impaired renal function, the use of certain oral hypoglycemic agents may be contraindicated. Biguanides such as metformin are contraindicated in patients with a creatinine clearance of less than 60 mL/min, due to the risk of lactic acidosis. Of interest, treatment with TKIs such as sunitinib and sorafenib have been observed to improve glycemic control in patients with diabetes mellitus.
Hyperlipidemia
Hyperlipidemia is frequently observed with mTOR inhibitors. Three percent of patients treated with temsirolimus or everolimus experienced grade 3 or 4 hyperlipidemia in phase 3 testing. Although patients who are treated with these agents may have limited life expectancies due to their underlying malignancy, many will live for at least several years, and may be at high risk from complications due to lipid abnormalities. Evaluation of lipids should occur at baseline and every 6 weeks if they are above recommended levels. Statins and bile-acid sequestering agents are first-line therapy for hypercholesterolemia, and for significant hypertriglyceridemia nicotinic acid or fibrates are indicated. Treatment of hypercholesterolemia and hyperlipidemia should follow standard guidelines. Caution should be used when considering agents to manage these metabolic toxicities, and certain statins are substrates of CYP3A4, so drug metabolism may be altered.
Thyroid Dysfunction
Hypothyroidism was reported in 14% of patients treated with sunitinib in the RCC phase 3 trial, though reported less frequently with pazopanib, and not reported with sorafenib in phase 3 testing. Retrospective evaluations of sorafenib and sunitinib suggest that the incidence of hypothyroidism is significant in certain populations. Hypothyroidism may contribute to other adverse effects, including fatigue and anemia. Evaluation of thyroid-stimulating hormone (TSH) and T4 at the initiation of therapy will provide a baseline. Thyroid dysfunction with sunitinib may result in changes between days 1 and 28 of each cycle, so monitoring at the beginning and end of 4-week cycles may be required in patients with symptoms consistent with hypothyroidism. Monitoring of TSH every 6 to 8 weeks during VEGF receptor–targeted therapy is necessary to monitor for treatment-related thyroid dysfunction. Thyroid replacement should be initiated for patients with TSH greater than 10 IU on day 1 of two consecutive cycles. The underlying pathophysiology of thyroid dysfunction remains somewhat unclear; many patients experience a brief mild drug-induced thyrotoxicosis, but an autoimmune etiology does not appear to be involved.
Renal effects
Renal dysfunction has been observed with sunitinib therapy, although in general renal toxicity has been mild. In a meta-analysis of 13 clinical trials with sunitinib, 65.6% of patients with kidney cancer experienced an increase in creatinine, with an RR of 1.35 ( P <.001), although the frequency of severe renal toxicity was low (<1%). Proteinuria, however, is observed in many patients receiving antiangiogenic agents, bevacizumab in particular. High-grade proteinuria was observed in 2.2% of patients (more common in patients with kidney cancer than other cancers [11.9%]) and may lead to nephrotic syndrome in some patients. In general, bevacizumab therapy is recommended to be held back when more than 2 g of protein are excreted in 24 hours, and potentially reinstituted when proteinuria has lessened. Use of an ACE inhibitor or an ARA may be appropriate, depending on the underlying kidney function, to reduce proteinuria and prevent progression of renal dysfunction, although there are limited data to support this with targeted therapy–induced proteinuria.
Dermatologic effects
Among the most common adverse events seen with these agents are cutaneous adverse events. Visible dermatologic toxicities can affect the patient physically, psychologically, and socially. These toxicities are particularly important to monitor because their manifestation can lead to poor adherence, suboptimal dosing, and discontinuation of an effective therapy.
The potential cutaneous toxicities seen with targeted therapies include hand-foot skin reaction (HFSR), rash, xerosis, pruritus, alopecia, erythema, skin discoloration, hair depigmentation, and subungual hemorrhages ( Table 2 ). HFSR is the most clinically significant of these dermatologic side effects. It is primarily seen with the multitargeted TKIs sorafenib and sunitinib, though less so with pazopanib. HFSR has not been described with the mTOR inhibitors temsirolimus and everolimus.
| Adverse Event | Overall Incidence (%) |
|---|---|
| Hand-foot skin reaction | 9–62 |
| Rash | 19–76 |
| Pruritus | 19–38 |
| Erythema | 16 |
| Alopecia | 27–53 |
| Hair depigmentation | 10–38 |
| Skin discoloration | 16–41 (sunitinib) |
| Subungual splinter hemorrhages | 25 |
| Xerosis | 16–31 |
HFSR is distinct from the hand-foot syndrome seen in patients receiving more conventional chemotherapy, such as, capecitabine or parenteral 5-fluorouracil. Classic hand-foot skin syndrome or palmar-plantar erythrodysesthesia (PPE) presents as symmetric, painful, red, edematous areas on the palms and soles. The HFSR associated with TKIs is more localized, and well-defined hyperkeratotic lesions are present. The pathogenesis of HFSR remains unknown. Platelet-derived growth factor (PDGF) and c-kit are present in epithelium of sweat ducts. Inhibitory effects of sunitinib and sorafenib on PDGF and of sunitinib on c-kit may play a role.
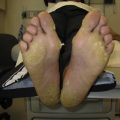
Symptoms of HFSR include tingling, burning, paresthesia, and/or dysesthesia on the palms and/or soles, and generally occur during the first 2 to 4 weeks of TKI therapy. Callous-like blisters (which usually do not contain fluid), erythema, dry or cracked skin, edema, desquamation, and hyperkeratosis are usually seen at pressure and flexor areas of the palms and/or soles. Severe and painful calluses can significantly affect a patient’s quality of life leading to a decline in function that may require discontinuation of therapy ( Fig. 1 ). In patients receiving sunitinib, symptoms of HFSR usually resolve during the 2-week break period.
To date, there are no evidence-based treatment guidelines on various topical remedies for HFSR. The management of side effects is based largely on clinical experience and advice from specialists. Prophylactic and supportive measures should begin before treatment starts. A full skin examination should be done prior to the start of therapy and at each visit. Patients with preexisting calluses may be predisposed to HFSR, and a pedicure is recommended. Application of an emollient-based moisturizer twice daily should begin before or at the beginning of treatment. If HFSR does develop, switching to a urea-based moisturizer may be helpful. Urea is a keratolytic that helps break down hyperkeratosis, and decreases epidermal thickness. Topical steroids, for example, clobetasol propionate cream, can be an effective palliative measure given its anti-inflammatory properties. Patients should be cautioned to avoid excess pressure or friction on affected areas. Avoidance of restrictive footwear and the wearing of gel insoles are advised. Wearing of cotton socks has been recommended. However, in their practice the authors recommend woolen socks, for example, SmartWool socks (SmartWool, Steamboat Springs, CO, USA), which can wick away sweat and reduce friction. Use of analgesics may be indicated to control pain. Some patients may be required to temporarily discontinue therapy or undergo dose reduction to alleviate symptoms. Once symptoms have resolved, the drug should be restarted at 50% of the starting dose. If, after careful monitoring, the toxicity does not recur, titration to full dose may be possible.
Of interest is a recent abstract presented at the 2011 Genitourinary Symposium. Michaelson and colleagues retrospectively evaluated 770 patients on sunitinib from 5 clinical trials to investigate correlations between the development of HFSR and efficacy end points. The investigators found that there was a strong correlation between the development of sunitinib-associated HFSR and improved clinical outcomes. If this finding is validated, patients and clinicians may be reluctant to reduce dose or temporarily halt therapy for fear of affecting efficacy.
The rash associated with targeted therapies can range from a mild erythematous flushing of the face to a more severe exfoliative dermatitis. The rash associated with the mTOR inhibitors tends to be milder than that seen with the TKIs, and is more maculopapular in appearance. Pruritus may or may not be present. Frequently the rash involves the face, scalp, and upper torso. Unlike the rash seen with endothelial growth factor receptor inhibitors, there is no known correlation with development of the rash and response to treatment.
As with HFSR, the absence of hard clinical data in the treatment of skin toxicities leads to a reliance on empirical evidence for treatment strategies. Papulopustular rashes can be treated with topical antibiotics, although in more severe cases systemic antibiotics may be required. Treatment with colloidal oatmeal lotion has been effective in treating acneform eruptions. If pruritus is present, antihistamines or “anti-itch” lotions, for example, Sarna, may be indicated. With sorafenib, these pruritic rashes frequently appear on the scalp, and betamethasone lotion may provide relief. Xerosis, with or without associated pruritus, can be relieved by the use of emollient-based moisturizers, such as, Aveeno and Eucerin. Again, the authors recommend patients begin using these moisturizers at the beginning of treatment with a targeted therapy. Patients should be educated to refrain from exposing the affected areas to hot water, as this may exacerbate symptoms.
Photosensitivity has been described with sunitinib, and patients should be advised to avoid prolonged sun exposure and use sunscreen. The skin discoloration seen in patients taking sunitinib usually presents as a yellowing or hypopigmentation of the skin. These changes may be distressful to the patient, who should be made aware of their potential appearance. Sunitinib and pazopanib have also been associated with hair depigmentation (gray or white). These changes resolve after discontinuation of the offending agent.
The appearance of subungual hemorrhage is unique to sunitinib and sorafenib. This condition is not associated with pain or change in nail integrity, and requires no treatment.
Pulmonary toxicity
Noninfectious pneumonitis a class effect of mTOR inhibition. Radiographically it appears as ground-glass opacities and nonmalignant lung consolidations. Patients may be asymptomatic or present with cough, dyspnea, or fatigue. To date, pneumonitis has been seen more often with everolimus. In the RECORD-1 trial of everolimus, an incidence of 13% was reported. White and colleagues presented data from an independent, blinded, retrospective radiological review of the same study and found an incidence of clinical pneumonitis of 13.5%. However, 38.9% of patients without clinical pneumonitis had new radiographic findings, compatible with pneumonitis. There are similar data with temsirolimus. The Global Advanced Renal Cell Carcinoma trial did not distinguish pneumonitis as an adverse event, although 26% of subjects developed a cough. These early data lead the authors to believe that the prevalence of noninfectious pneumonitis may be higher than is appreciated. At present the etiology of pneumonitis and mTOR inhibition is unknown.
Careful clinical monitoring for this adverse event should be done at each visit ( Box 1 ). Pretreatment pulmonary function tests (PFT) may be needed, especially if the patient has a history of pulmonary disease. A chest computed tomography scan should be performed on any patient exhibiting new respiratory symptoms. Recommended management is outlined in Table 3 .
CYP3A4 Inhibitors
Amiodarone
Aprepitant
Atazanavir
Clarithromycin
Cyclosporin
Darunavir
Diltiazem
Fluconazole
Grapefruit juice
Indinavir
Itraconazole
Ritonavir
Saquinavir
Telithromycin
Verapamil
Voriconazole
CYP3A4 Inducers
Carbamazepine
Dexamethasone
Efavirenz
Nafcillin
Oxcabazepine
Phenobarbital
Phenytoin
Primidone
Rifampin
a List is not inclusive. Available at: http://medicine.iupui.edu/clinpharm/ddis/table.asp . Accessed January 22, 2011.