Series
Levels dissected
Sivanandan and Soo (2001) [37]
II–IV
Pingpank et al. (2002) [38]
II–V
Caron et al. (2006) [34]
III, IV
Yanir and Doweck (2008) [39]
II–V
Kupferman et al. (2008) [40]
II–V
Farrag et al. (2009) [31]
IIa, III, IV, Vb
Yuce et al. (2010) [41]
II–V
Grant et al. (2010) [30]
III, IV, Vb
Lim et al. (2010) [32]
II–IV
Ahmadi et al. (2011) [33]
II–IV
Dissection of level I is unnecessary unless clinical evidence of disease supports it. It is universally agreed that dissection of levels III and IV is necessary. Most surgeons support dissection of level II. Grant and co-workers are of the opinion that only a modest extension of the thyroidectomy incision is necessary to dissect levels III, IV and Va [30]. Treatment of level II requires a significantly larger incision and exposure. They express concern over the relatively high complication rates associated with more extensive neck dissections. Level II is addressed by these workers when clinical or radiological disease is evident. In their series of 420 patients studied over a 7-year period, Grant and co-workers identified a relapse in level II in only one patient [30]. In Farrag and associates’ experience, level IIb is never involved in isolation and they only address the area above and lateral to the accessory nerve if level IIa is involved [31]. The prospect of shoulder dysfunction from the dissection of the accessory nerve has limited the dissection of level V by some investigators [30–33]. Ahmadi and co-workers noted that level V was never involved in isolation [33]. When treating level V they limit dissection in the posterior triangle to the area below the accessory nerve. The accessory nerve and its course in the posterior triangle are identified to avoid injury to the nerve; however, circumferential dissection is avoided. Lim et al. also emphasized that level V is not involved in isolation, and only in the presence of positive nodes in level IV [32]. They dissect level V only if histological evidence confirms involvement of level IV. Caron and co-workers target levels III and IV. Level II is addressed with extensive involvement of level III and level V is dissected only in the presence of clinical or radiological evidence of disease [34]. They have been able to achieve good disease control.
The Authors’ Approach
Lymph nodes in the lateral neck in WDTC are treated with a selective neck dissection of levels IIa, III, IV and Vb, in keeping with current guidelines [35]. Histological confirmation is recommended before proceeding with a selective lateral neck dissection. In the experience of the present authors, most palpable nodes in patients with PTC are positive. In patients presenting primarily with lateral neck disease, the selective lateral neck dissection is done in conjunction with a total/near-total thyroidectomy and a central compartment dissection. When the lateral neck is treated remote from the initial thyroidectomy, addressing the central compartment is individualized to the patient. In this situation, judgement regarding reoperation in the central compartment involves reviewing the details of the initial operation, including the management of the paratracheal lymph nodes, the recurrent laryngeal nerve and the parathyroids. The functional status of the parathyroids and recurrent laryngeal nerve needs to be assessed. Careful attention is paid to the status of the central compartment with preoperative imaging, using both ultrasound and CT, and intraoperative assessment.
Technique
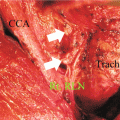
The procedure is performed under general anaesthesia without paralysis to facilitate identification of nerves. A nerve stimulator should be available. Exposure for this dissection can be achieved by extending the thyroidectomy incision laterally and superiorly at its posterior extent, if necessary. The use of a separate transverse incision superiorly (McFee incision) may facilitate exposure in the long neck and with the involvement of upper neck nodes. Skin flaps are elevated in the subplatysmal plane. It is important to consider that the platysma thins out in the posterior triangle and that the accessory nerve runs a superficial course. The anterior boarder of the trapezius is exposed. The accessory nerve (AN) is identified. One of the authors (R.W. Nason) uses the nerve point in the posterior triangle [36]. An imaginary line is drawn from the thyroid notch to the point at which the sensory nerve emerges from behind the posterior board of the sternocleidomastoid muscle (SCM). The midpoint of the SCM approximates this point. The AN enters the posterior triangle within 2 cm above this line, and exits posteriorly beneath the trapezius muscle within 2 cm below. A haemostat is used to spread dense fascia and areolar tissue along the posterior border of the SCM to identify the AN where it enters the posterior triangle. The course of the nerve is identified without circumferential dissection. The dissection is started inferiorly dividing the distal external jugular vein and transecting the lymphoareolar tissue between ties until the plane of dissection is established on the deep layer of cervical fascia. The lymphoareolar tissue below the course of the AN is mobilized from the anterior board of the trapezius and immediately on the posterior compartment muscles, which include the splenius capitis, levator scapulae and the scalene muscles. With dissection on the medial board of the levator scapulae, the cervical plexus is encountered. These cutaneous sensory branches are divided in the presence of bulky disease. With minimal node involvement they can be preserved. The phrenic nerve is identified on the surface of the anterior scalene muscle. The SCM is mobilized from the carotid sheath and retracted cephalad and medially. Medial retraction of the specimen exposes the internal jugular vein. The specimen is then mobilized from the vein with sharp dissection and subsequently from the carotid artery with attention to the course of the vagus nerve. The specimen is passed underneath the SCM and with the muscle retracted laterally and inferiorly the dissection of the carotid sheath is completed in the anterior triangle. The dissection of the upper carotid sheath is completed above the level of posterior belly of digastric. If it is necessary to continue superiorly to the skull base (level IIb dissection), the hypoglossal nerve is identified and followed superiorly to identify the proximal internal jugular vein and the accessory nerve in the anterior triangle before it enters the SCM [36]. It is generally not possible, or for that matter necessary, to maintain continuity between the resected thyroid and lymphoareolar contents of the central compartment and lateral compartment.
Commentary on Chaps. 2, 3 and 4
Janice L. Pasieka
What to do with the lymph nodes in well–differentiated thyroid cancer
Recently, the management of the lymph node compartments in well-differentiated thyroid cancer (WDTC) has been the focus of much debate among the surgeons and endocrinologists dealing with this disease. Why has there been so much recent ‘press’ on this topic? Much of this has to do with the changing paradigm in the definition of thyroid cancer recurrence. Until a decade ago the identification of recurrence in WDTC relied on physical examination and/or the demonstration of disease on follow-up radioactive I131 scans. This has changed with the advent of high-resolution ultrasounds and the measurement of stimulated thyroglobulin (Tg) levels. These technological advances have allowed for the identification of disease that was too small to detect by physical examination and I131 scanning. As such, the definition of persistent and recurrent disease has changed in recent years. Because of this, endocrinologists, medical oncologists and surgeons have questioned whether more extensive surgical removal of the disease at the first operation would benefit this patient population—hence the development of prophylactic central neck dissections (pCLNDs).
In this monograph, Pathak and Nason have described their approaches to both the central and lateral neck compartments in WDTC. The American Thyroid Association (ATA) set out to provide guidance on the issue of pCLND, yet recognized early that uniformity in definitions was needed to interpret the current literature. Carty et al. defined the central neck and introduced definitions for ipsilateral and bilateral level VI dissections [1]. It is important that surgeons operating for thyroid cancer are familiar with these standardized definitions. Level VI includes the tissue between the carotid sheaths from the hyoid bone to the innominate artery. This includes the pyramidal lobe, forgotten by many, the anterior peritracheal tissue and the tissue deep in the neck in which the inferior parathyroid gland and recurrent laryngeal nerve (RLN) lie. It is because of the close proximity of the RLN to the parathyroid glands that pCLND should not be taken lightly [2, 3]. Although risk to the RLN appears to be similar to that from total thyroidectomy alone, hypoparathyroidism is increased, especially when bilateral pCLNDs are performed [2, 3]. The clinical benefit of removing clinically negative nodes (cN0) from this compartment has been discussed extensively in the literature. Most authors would agree that to date no survival benefit is seen in patients undergoing a pCLND for WDTC [4]. Some have argued that CLND decreases postoperative serum thyroglobulin (Tg) levels, thus decreasing recurrence/persistence rates [5]. The oncological principle of removing the draining lymph node basin for most cancers is to stage the patient accurately, and as such, influence postoperative therapy. Forty per cent of patients with cN0 disease were up-staged as a result of the histological findings on pCLND. Not surprising, given the high incidence of positive lymph nodes in WDTC. However, in centres that provide selective I131 ablative therapy, pCLND has led to an increased utilization of I131 therapy in 33 % of WDTC patients [6]. Many centres utilize a more selective use of I131 therapy in low-risk patients because of the lack of survival benefit seen with its use [7]. Thus, for the individual surgeon dealing with WDTC, the need for pCLND must be individualized to that surgeon and their referral cancer centre. First, the surgeon must know his/her rate of RLN injury and hypoparathyroidism with CLND. Second, the surgeon has to understand how the additional information from the central lymph nodes will be utilized. If their centre routinely gives I131 to all patients regardless of the risk status, then pCLND may be of little benefit. If the centre selectively gives therapy to all N1 disease, providing the additional staging information gained from the pCLND may be of some benefit. Yet clinically, most clinicians recognize that there are varying degrees of magnitude in the risk for recurrence for N1 disease. The upstaging of low-risk patients with small-volume nodal disease conveys a much smaller risk of recurrence than those with large-volume disease. Randolph et al. have recently proposed an N1 stratification for the use of I131 therapy [8]. Their document provides a rational approach to the question of how to utilize the information provided from a CLND.
A prospective, randomized controlled trial of pCLND in WDTC would help clarify this issue for the surgeons. However, given the low rates of both structural recurrence and morbidity after surgery for cN0 disease, prohibitively large sample sizes would be required for sufficient statistical power to demonstrate significant differences in outcomes [9]. Biochemical molecular tumour markers are gaining wider use in clinical practice and in time, hopefully, will provide more specific information on which surgical decision-making can be based [4, 10].
Clinically positive lymph nodes warrant a compartmental dissection, as outlined by Pathak and Nason in this monograph. The utilization of preoperative lymph node mapping with ultrasound has become the standard of care in most North American centres. Recent changes in the ATA guidelines for medullary thyroid cancer have moved from routine lateral neck dissections to selected dissections of the lateral compartments on the basis of lymph node mapping [11]. Although this approach relies on the sensitivity of thyroid ultrasonography, surgeons, endocrinologists, along with radiologists are becoming more skilled with this adjunct in the clinical evaluation of the thyroid and its regional lymph node basins. Most clinicians have expanded the role of ultrasound lymph node mapping with fine-needle aspiration confirmation to WDTC. This approach helps prepare both the patient and the surgeon preoperatively.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree