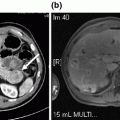
Fig. 15.1
Axial CT scan slice showing gallbladder wall enhancement, suspected wall discontinuity (red arrow) and intra-luminal air (black arrows)
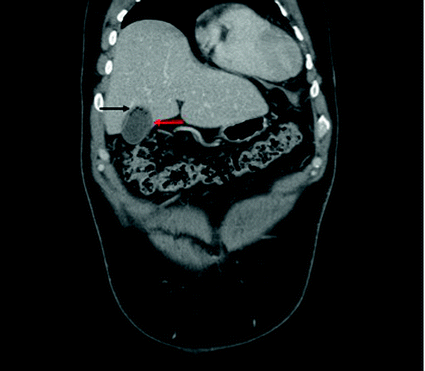
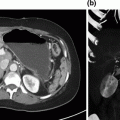
Fig. 15.2
Coronal CT scan slice showing gallbladder wall enhancement, suspected wall discontinuity (red arrow), and intra-luminal air (black arrow)
Surgical management in the form of cholecystectomy was recommended to the patient. A laparoscopic approach was attempted; however conversion to an open cholecystectomy ensued, due to the finding of a thin-walled necrotic gallbladder, poor handling of the organ rendering dissection challenging, in combination with poor visualization due to the patient’s body habitus.
The gallbladder was removed, and a closed-suction drain was placed within the gallbladder fossa. Postoperatively, the patient required ongoing aggressive fluid therapy to address an acute kidney injury, maintain urine output, and optimize hemodynamics. As expected, the inflammatory response moderated itself between 48 and 72 h, allowing clinical amelioration. Other minor complications such as atelectasis and poor glycemic control were improved via standard therapies. The patient was discharged home on postoperative day 5. His drain was removed prior to discharge in light of the low-volume, sero-sanguinous effluent.
Our Approach
Although acute cholecystitis (AC) represents a common diagnosis within any General Surgery practice, GC often causes significantly more angst due to the technical challenges and increased complications [1]. GC is typically defined as necrosis with possible perforation of the gallbladder wall caused by ischemia following progressive vascular insufficiency [2]. GC has also long plagued general surgeons (first reported in 1894 [3]) and continues to be encountered in up to 40% of patients presenting with AC [2–5].
Acute GC should be viewed as a surgical emergency. This recommendation is especially true given its association with increased morbidity, including bile duct injuries [4]. Furthermore, delays in management result in inferior outcomes [1]. “Gangrene” of the gallbladder wall reflects the duration of the inflammatory and ischemic process. More specifically, obstruction of the cystic duct results in prolonged increased intra-luminal pressure, to the point where this pressure exceeds the arterial inflow pressure, causing ischemia of the organ [1, 5].
Given this well-defined pathophysiology, timely diagnosis and treatment must be engaged to limit morbidity [1]. We present a case of GC to highlight the salient features of this disease, notably the challenges and delays surrounding its diagnosis in at-risk patients. We also review optimal management strategies, and offer insight into approaching this onerous clinical scenario. With a relative paucity of current literature on this topic, combined with the decreased comfort of contemporary general surgeons performing open cholecystectomy (which is more frequently required in this scenario), surgeons facile with the liver and extra-hepatic biliary tree will not infrequently be called upon to assist in the care of these patients.
Initial Presentation
As outlined in the preceding case, delays in presentation, and further delays in obtaining diagnostic imaging and consultation by a surgical service, are more frequent among individuals with GC [6]. Numerous risk factors of varying significance, listed below, have been reported to predispose patients to progress to GC [1, 2, 5, 7–13]. Abnormalities in laboratory values often include [5] leukocytosis, and an elevation in alkaline phosphatase and GGT. Increases in transaminases can also be noted, driven by hepatocyte necrosis in the liver tissue adjacent to the gallbladder bed [2]. As the inflammatory process progresses, the edematous or hydropic gallbladder can display a mass effect on surrounding structures, such as the bile duct, and trigger a rise in bilirubin [2]. This situation further complicates the clinical picture and may delay treatment as investigations are pursued to clarify the hyperbilirubinemia. Despite, and in light of, these various changes and patient-related factors, preoperative prediction of GC remains as low as 9% [6].
Risk Factors for Gangrenous Cholecystitis |
|---|
Increased age |
Male sex |
Diabetes mellitus |
Coronary artery disease |
Steroid use |
Dependent functional status |
SIRS (systemic inflammatory response syndrome) at presentation |
Gallbladder wall thickening, pericholecystic fluid on ultrasound |
Diabetes is consistently associated with GC [1, 2, 4, 5, 8–13]. The pathophysiology of this association is thought to be two-fold. Diabetes leads to micro-vascular disease [3] and therefore, smaller caliber, atherosclerotic vessels may have increased susceptibility to the increased pressure stemming from cystic duct occlusion and frank ischemia may take place earlier in the process of cholecystitis. Second, diabetes has a known associated neuropathy, which may limit the sensory perception of an inflamed gallbladder [3]. These combined diabetes-related issues subsequently cascade into delayed presentation, as well as altered presenting symptoms, typical of patients with GC. Not surprisingly, diabetic patients also may be subject to higher rates of perioperative morbidity and mortality [5].
Elderly patients tend to present in a similar fashion. Impaired sensation of symptoms, relative inability to mount an inflammatory response, combined with possible changes in cognition and communication all contribute to delayed or missed diagnosis and treatment [2]. Institutional reviews of elderly patients with GC have identified higher rates of mortality [5, 8]. Patients for whom mortality occurred also were noted to have increased delays in time to admission (54 vs. 21 h) [2]. As exemplified in this case, the typical patient at risk of GC is male, diabetic, and of advanced age. These three factors are simply surrogates for less healthy patients who present later in the course of their disease, and consequently with a more severe cholecystitis [3].
Diagnostic Imaging
Patients suspected to have cholecystitis will typically have a leukocytosis, and often abnormalities in liver enzymes [1, 5, 7]. The diagnostic imaging modality of choice remains the ultrasound (readily available, safe, and low in cost) [14, 15]. Despite the user dependence associated with ultrasonography, its sensitivity in detecting acute inflammation of the gallbladder is reported to be 90–95% [5]. However, the ability of ultrasound to discriminate between gangrenous and non-gangrenous cholecystitis is quite limited [6]. This inability to differentiate these two entities is true of diagnostic imaging in general [1]. Gallbladder wall thickening, pericholecystic fluid, and a positive sonographic Murphy’s sign are all reported to be associated with GC [4, 5]. These findings are also present in simple acute cholecystitis, and comprise the diagnostic criteria in the revised Tokyo guidelines [16].
Although ultrasonography remains the gold standard for acute cholecystitis, CT may offer a diagnostic advantage over ultrasound in detecting GC. The presence of a perfusion defect, or discontinuous/irregular enhancement of the gallbladder mucosa, has a high positive predictive value (94–100%), sensitivity of 30–70%, specificity of up to 100%, and accuracy of 80% [7, 17, 18]. Often, the diagnosis of acute cholecystitis will be suspected clinically, and confirmed by ultrasound. However, a minority of patients will undergo cross-sectional imaging, which may explain the relatively high intraoperative, rather than preoperative, discovery of GC.
Magnetic resonance (MR) has proven to be an effective modality for imaging of the biliary tree. MR can identify features of GC, such as ulceration, hemorrhage, necrosis, or micro-abscesses, to a greater degree than US or CT, notably when intravenous contrast is employed [18]. As with the previous modalities, the finding of inhomogeneous wall enhancement or disrupted mucosal enhancement on MR is characteristic of GC [18]. While air in the gallbladder lumen, emphysematous cholecystitis (EC), was observed in our case, this finding is unusual in patients with GC.
Given that most patients undergo ultrasound assessment as their sole imaging modality, clinical acumen must be relied upon to suspect GC and avoid missing or delaying the diagnosis. If suspicion is high, other modalities such as CT or MR can be utilized to confirm the diagnosis. However, treatment options may not be altered by these additional tests, as these patients typically require surgical intervention. The sagacious surgeon will expedite surgery, anticipate an intraoperative diagnosis of GC, and prepare the perioperative team accordingly. Furthermore, if GC poses a challenging resection, involvement of a hepatobiliary surgeon may be helpful.
Tokyo Guidelines
An international group of experts has published guidelines to better characterize and guide the management of cholecystitis and cholangitis. These Tokyo Guidelines (TG) highlight the process of cholecystitis as it evolves from an edematous stage (1st stage), to necrotizing cholecystitis (2nd stage), and then finally to suppurative cholecystitis (3rd stage) [19]. The diagnosis of GC lies between the second and third stages, where findings such as hemorrhage and necrosis result from vascular occlusion, which then progresses to necrosis, breakdown of the gallbladder wall, and development of intra-mural or pericholecystic abscesses [20].
These stages are either established intraoperatively or via pathologic confirmation. Preoperatively, patients are categorized according to clinical grade. As part of the 2012 Tokyo Guidelines, [16] proposed severity assessment criteria identify three grades (mild, moderate, severe) based on presentation and duration of symptoms, laboratory values, and associated organ/system dysfunction. Management options are tailored to the presence of these grades. As previously mentioned, predicting GC is challenging and, as such, management should be responsive to the patient’s clinical picture.
In the presented case, the presence of leukocytosis above 18,000/mm3, duration of symptoms >72 h, and the presence of marked inflammatory changes on imaging categorize the patient as Grade II—moderate cholecystitis. This prompted early cholecystectomy, in accordance with current (TG 13) guidelines [21].
Clinical Pearls
- 1.
If clinically appropriate, surgical management should be expedited if GC is suspected (with a surgeon facile in challenging biliary diseases and anatomy).
- 2.
Perform a bile duct time-out in the setting of ambiguity (B.E. S.A.F.E.).
- 3.
Possess a low threshold to convert to an open procedure for safety and to ensure a total cholecystectomy.
- 4.
Beware of nearby hepatic venous branches.
- 5.
Follow curve of gallbladder to decrease risk of injury to bile duct.
Management
Initial management is directed toward fluid resuscitation, and the institution of antimicrobial therapy if sepsis is suspected. Antibiotic regimens, such as a broad-spectrum penicillin, should treat common biliary organisms including Enterococcus and Gram-negative rods. Up to one-third of patients with GC and EC also are likely to harbor anaerobes including Clostridia perfringens. Patients are nil per os (npo), provided with adequate analgesia, and urgent steps are taken to treat the cholecystitis [14].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree