Appropriate axillary staging is a critical component of breast cancer care. In addition to being an important prognostic factor, nodal status and the extent of nodal disease affects decisions regarding surgical management as well as recommendations about systemic therapy and adjuvant radiation.
As recently as the early 1990s, axillary status was determined by axillary lymph node dissection (ALND) for all breast cancer patients regardless of clinical nodal status. Although ALND is accurate in identifying lymph node metastases and staging the axilla, it is also associated with potential morbidity including lymphedema, shoulder dysfunction, pain, and paresthesias.1,2 In addition, ALND identifies nodal metastases in only 20% to 35% of women who present with clinically node-negative disease.3–5 Because removing healthy lymph nodes provides no benefit, a more selective approach for axillary staging was required. Sentinel lymph node (SLN) dissection (SLND), which allows for accurate nodal staging with less morbidity than ALND, represents such an approach.2,6,7
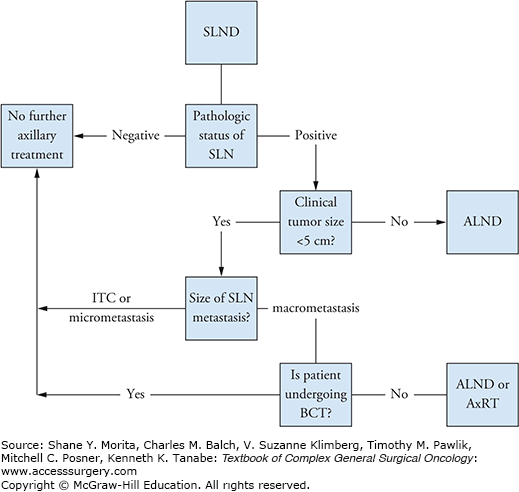
The SLND procedure has been validated in numerous single-institution and multicenter studies and prospective randomized trials.5,8–11 For patients with clinically node-negative disease, SLND is now standard for axillary evaluation, and ALND can be omitted in patients with a negative SLN without diminishing disease-free survival (DFS), overall survival (OS), or local-regional control. Although completion ALND in patients with a negative SLN was abandoned soon after the adoption of the SLND procedure, ALND continued to be recommended for patients with a positive SLN. However, this practice has been called into question, and recent studies have shown that highly select patients with a positive SLN can also be spared ALND.12,13 The goals of this chapter are to discuss the clinical staging of the regional lymph nodes in breast cancer patients, review the SLND procedure, and discuss clinical trial results guiding the treatment of patients with clinically node-negative disease. An algorithm for managing patients with clinically node-negative breast cancer is shown in Fig. 78-1.
FIGURE 78-1:
Algorithm for management of patients with clinically node-negative breast cancer. SLND, sentinel lymph node dissection; SLN, sentinel lymph node; ALND, axillary lymph node dissection; ITC, isolated tumor cell; BCT, breast-conserving therapy (breast-conserving surgery plus whole-breast irradiation); AxRT, axillary radiotherapy.
Determining whether the tumor has spread to the local-regional lymph nodes is an important step in the initial staging of breast cancer. It provides prognostic information and helps determine appropriate management strategies. The clinical nodal stage is based on physical examination and radiologic studies. When performing a physical examination of the regional lymph nodes, if adenopathy is identified, the size of the palpable nodes should be noted. If axillary adenopathy is identified, whether the lymph nodes are mobile or matted should also be recorded. Physical examination is limited, however, in that lymph nodes with metastatic foci may not be palpable and nodes may become palpable after breast biopsy owing to a reactive process. Older series report false negative rates ranging from 30% to 45% for physical examination.14–16
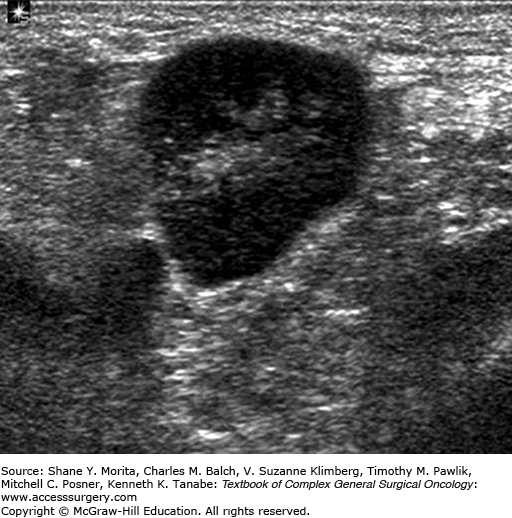
Given its limitations, physical examination may be complemented by high-resolution ultrasonography. Ultrasonography is favored over other imaging modalities for evaluating regional lymph nodes because it is highly accurate and provides easy access for obtaining a tissue biopsy.17 Ultrasonography can be used to assess lymph nodes for alterations in size, shape, and contour as well as changes in cortical morphology and texture. Sonographic features that are suspicious for metastatic disease include enlarged size, the presence of a thickened or eccentric cortex, and compression or displacement of the fatty hilum (Fig. 78-2).17–19 Suspicious-looking lymph nodes are subjected to fine needle aspiration biopsy (FNAB) to obtain a tissue sample for pathological analysis to determine definitively whether metastatic disease is present. FNAB has a reported sensitivity of approximately 90% and a specificity of as high as 100%.20,21 Core needle biopsy has similar sensitivity and specificity and can also be used.22
The clinical nodal stage is assigned according to the American Joint Commission on Cancer (AJCC) staging system, which has different parameters for nodal staging depending on whether clinical or pathological evaluation is used.23 Patients without regional lymph node metastases identified by physical examination or imaging studies are staged as clinical N0. Axillary lymph node disease is designated as clinical N1 disease if the lymph nodes are mobile and in the ipsilateral nodal basin or as clinical N2 if the lymph nodes are fixed or matted. The clinical N2 designation is also used for clinically apparent internal mammary nodes in the absence of clinically evident axillary metastases. Metastases in the internal mammary lymph nodes in the presence of axillary metastases or metastases in the infraclavicular or supraclavicular lymph nodes are classified as clinical N3.
Patients with clinical N0 disease are candidates for SLND. SLND is predicated on the concept that the breast has an orderly lymphatic drainage pattern with specific lymph nodes, the SLNs, draining the breast first followed by drainage to the remainder of the nodal basin.
The concept of SLND in breast cancer was first reported in 1994 by Giuliano and colleagues, who performed SLND followed by ALND in 174 patients.24 The authors used isosulfan blue dye injected peritumorally to map the lymph nodes. A positive SLN was identified in 114 (65.5%) of 174 procedures and accurately predicted the axillary nodal status in 109 (95.6%) of 114 cases. The authors concluded that lymphatic mapping could accurately identify the SLN and that SLND could enhance staging accuracy while potentially avoiding the need for ALND. The SLND procedure has subsequently been evaluated in multiple single-institution and multicenter studies, which have shown that experienced surgeons can identify an SLN in 93% to 99% of clinically node-negative patients with a false-negative rate of less than 10%.5,10,25–27
While initial studies evaluating SLND included primarily patients with small, unifocal, invasive tumors, the technique has since proven efficacious in multiple scenarios, including in patients with ductal carcinoma in situ, larger tumors, multicentric or multifocal disease, male breast cancer, and breast cancer in pregnancy and in patients who have undergone prior breast or axillary surgery.28,29 Thus, SLND is standard practice for virtually all patients with clinical T1–3, N0 breast cancer.
Several technical aspects must be considered when performing SLND. One of these aspects is the choice of whether to use blue dye, a radioactive tracer, or both for lymphatic mapping (Fig. 78-3). Whereas Giuliano et al24 used isosulfan blue dye in their initial study of SLND, in another early study, Krag et al30 used a radioisotope and handheld gamma probe to identify the SLN. Subsequently, investigators used the combination of blue dye and radioisotope for lymphatic mapping.
In a multicenter registry study that included data provided by 99 surgeons on more than 800 patients, McMasters et al31 reported that SLN identification rates did not differ significantly when a single tracer (86%) or dual tracers (90%) were used. In patients in whom both tracers were used, a greater mean number of SLNs was identified (2.1 vs. 1.5; p < 0.0001) and a lower false-negative rate was found (5.8% vs. 11.8%; p < 0.05). In a meta-analysis evaluating different lymphatic mapping techniques, the SLN identification rate was 83.1% with blue dye alone, 89.2% with radioisotope alone, and 91.9% with dual tracers, and the false-negative rates were 10.9%, 8.8%, and 7.0%, respectively.32 On the basis of the inclusion dates, both the study by McMasters et al and the meta-analysis likely include data reflecting many surgeons’ initial experience. Taken together, these data suggest that the combination technique is most accurate for surgeons with less experience with SLND. A recent study from the University of Texas MD Anderson Cancer Center suggests that once a surgeon has extensive experience with SLND, either technique will result in excellent SLN identification rates. We investigated SLND in 3171 patients with clinically node-negative breast cancer and found SLN identification rates of 97.5% when a single-tracer technique was used and 99.0% when a dual-tracer technique was used.25 In that study, 542 patients had a planned completion ALND, and in that cohort, the false-negative rate was 4.1%. Factors associated with a false-negative event were using blue dye alone for the lymphatic mapping (p < 0.0001) or removing only one SLN (p = 0.001).
The tracer injection site is another technical aspect of SLND that has been a topic of some discussion. Whereas Giuliano et al injected the tracer peritumorally in their initial study of SLND, in another early study, Veronesi et al33 injected the tracer subdermally and reported an SLN identification rate of 98.2% and a false-negative rate of 4.7%. Rodier et al34 conducted a prospective, multicenter study in which both blue dye and a radioisotope were used and found that the SLN identification rate in patients in whom the tracers were injected in a peritumoral location was not significantly different from that in patients in whom the tracers were injected in a periareolar location. In addition, the rates of concordance of the blue SLNs and the most radioactive SLNs (“hot” nodes) by injection site did not differ significantly. Based on this and other studies showing similar SLN identification rates using peritumoral or subareolar injections,35,36 it is now accepted that multiple techniques are appropriate and that surgeons should use the technique with which they are most comfortable.
Some caveats must be considered when selecting the injection site for SLN mapping, however. For patients with multifocal or multicentric breast cancer, many surgeons advocate using a subareolar injection.37,38 In addition, although the clinical importance of nonaxillary SLNs remains a matter of some debate, drainage to these sites may occur when a peritumoral injection is used (Fig. 78-4). In one MD Anderson study, for example, we found that approximately 25% of patients who underwent peritumoral injection followed by lymphoscintigraphy had drainage to extra-axillary lymph nodes, with the most common site being the internal mammary nodes.39 In a subsequent study of more than 1770 patients, lymphoscintigraphy revealed that 333 (18.8%) had internal mammary drainage, which multivariate analysis revealed to be significantly associated with worse distant DFS but not local-regional recurrence (LRR) or OS.40 In a smaller study of 71 patients in whom internal mammary SLNs were identified and resected we found that 11 patients had a positive internal mammary SLN and that in 7 of these 11 patients, the internal mammary SLN was the only positive SLN, thereby altering recommendations for adjuvant therapy.41 We acknowledge that these rates of internal mammary SLN metastases are low, and at our institution, the technical aspects of SLND, including decisions regarding single or dual tracer use, the site of tracer injection, and the use of lymphoscintigraphy are left to the discretion of the attending surgeon.
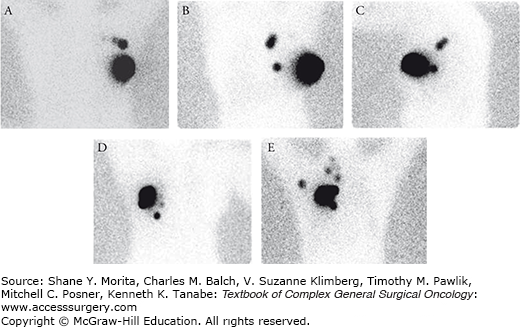
FIGURE 78-4:
Drainage patterns after peritumoral injection of radioactive tracer. When the tracer is injected peritumorally, lymphoscintigraphy may identify drainage to axillary lymph nodes as well as nonaxillary nodal basins. A. Anterior image demonstrating drainage to two axillary sentinel lymph nodes (SLN). B. Anterior image showing drainage to three internal mammary SLNs and faint activity corresponding to an axillary SLN. C. Lateral image obtained from the same patient shown in image B showing the axillary SLN. D. Anterior image identifying drainage to the internal mammary chain with the dominant node located inferiorly along the internal mammary chain at the level of the sternal tip. No appreciable axillary drainage was identified. E. Anterior image demonstrating multifocal drainage to axillary, supraclavicular, and internal mammary regions.
Another consideration of SLND is the number of lymph nodes that must be resected. SLND requires that all blue, “hot,” and suspicious-appearing lymph nodes be excised. With respect to “hot” lymph nodes, most surgeons follow the 10% rule, first described by Martin and McMasters,42 which dictates that all SLNs with radioactive counts greater than 10% of that of the “hottest” radioactive node be removed. In most patients, one to three SLNs are removed during SLND.
Multiple studies have shown that removing two or three SLNs identifies 93% to 99% of patients with nodal metastases.43–45 A combined analysis from the University of Michigan and the Mayo Clinic revealed that removing three SLNs identified 98% of patients with nodal metastases and removing four SLNs identified 100% of patients with nodal metastases. In the Axillary Lymphatic Mapping Against Nodal Axillary Clearance (ALMANAC) trial, removing the first four SLNs identified 99.6% of patients with nodal metastases.46 Taken together, these studies’ data suggest that removing more than four SLNs provides little benefit in the way of identifying patients with nodal metastases. Rather, removing two to four SLNs appears to be optimal for identifying nodal metastases and minimizing the false-negative rate. In the ALMANAC trial, the false-negative rate was 10% when one SLN was removed but only 1% when three or more SLNs were removed. Similarly, in the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-32 trial (discussed in detail below; see the section “Management of the SLN-Negative Patient”) the false-negative rate was 17.7% when one SLN was removed but much lower—5.5%—when four SLNs were removed.4
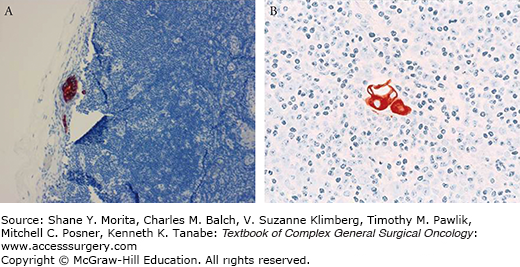
One purported benefit of SLND is that it allows for a more rigorous pathological evaluation of fewer lymph nodes that are at the highest risk for having metastatic disease. Consensus statements from the College of American Pathologists (CAP) and the American Society of Clinical Oncology (ASCO) recommend that SLNs be processed in sections no greater than 2.0 mm along the longest axis and that all sections be subjected to microscopic examination.47,48 Hematoxylin and eosin (H&E) staining of these step-sections should detect virtually all metastatic disease.49,50 Although the ASCO and CAP guidelines do not recommend the routine use of immunohistochemistry (IHC) staining for cytokeratin, many pathologists still choose to perform IHC staining to evaluate SLNs. The addition of IHC staining to H&E staining identifies occult metastases in approximately 10% to 15% of patients (Fig. 78-5).3,51 To address the increasing detection of small-volume metastases, when the AJCC revised its staging system in 2002, it established definitions of macrometastasis (>2.0 mm), micrometastasis (>0.2 to 2.0 mm), and isolated tumor cells (ITC; ≤0.2 mm).52 The pathological categories pN1mi and pN0(i+) were also added to indicate the presence of micrometastases or ITC. The 7th edition of the AJCC staging system further refined the definition of ITC to be a tumor cell cluster less than or equal to 0.2 mm or the presence of fewer than 200 cells in a single histologic cross section.23 ITCs may be detected by either H&E or IHC staining.
The significance of small-volume disease has been debated and a number of large trials have investigated its role in the management of breast cancer. The American College of Surgeons Oncology Group (ACOSOG) Z0010 trial prospectively evaluated occult metastases in the SLNs and bone marrow of 5210 patients with T1–2, clinically node-negative breast cancer who underwent breast-conserving surgery and whole-breast irradiation (WBI) between 1993 and 2003.3 An SLN was identified in 5119 patients (98.3%), including 3904 patients (76.3%) with SLNs that H&E evaluation revealed to be negative. Of the negative SLNs, 3326 were assessed by IHC in a central laboratory, and 349 (10.5%) were found to have occult metastases. Because the treating physicians were blinded to the results of the IHC evaluation, their decisions regarding adjuvant systemic therapy were dictated by the characteristics of the primary tumor and the results of the H&E evaluation of the SLN. At a median follow-up of 6.3 years, the ACOSOG investigators reported that IHC evidence of occult metastases had no significant association with recurrent disease or death. The 5-year DFS rates were 92.2% for patients with IHC-negative SLNs and 90.4% for patients with IHC-positive SLNs (p = 0.82), and the 5-year OS rates were 95.7% and 95.1%, respectively (p = 0.64). Similarly, in the NSABP B-32 trial, in which patients with clinically node-negative breast cancer were assigned to undergo either SLND plus ALND or SLND with ALND performed only if the SLN showed metastatic disease, SLNs found to be negative by H&E evaluation by local laboratories were sent to a central laboratory for IHC analysis.51 Of the 3884 patients who had SLNs found to be negative by H&E and for whom follow-up data and SLN blocks for IHC were available, 616 (15.9%) had occult metastases, including 430 (11.1%) with ITCs, 172 (4.4%) with micrometastases, and 14 (0.4%) with macrometastases. Although the estimated 5-year OS and DFS rates of patients with occult metastases (94.6% and 86.4%, respectively) were significantly lower than those of patients without occult metastases (95.8% and 89.2%, respectively; p = 0.03 and 0.01, respectively), these differences were likely due to the study’s large sample size. The absolute differences in the two groups’ OS rates (1.2%) and DFS rates (2.8%) are not likely clinically relevant.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree