Adjuvant treatment options for stage I seminoma include surveillance, radiation, and hemotherapy. Despite excellent results for both adjuvant chemotherapy and radiotherapy, many concerns have been raised in regards to the potential long-term toxicities of these treatments. To minimize the burden of treatment, there has been a shift away from adjuvant treatments for stage I testicular seminomas toward surveillance protocols for seminoma survivors. This article reviews the evidence for all adjuvant treatment options for stage I testicular seminomas with a particular focus on surveillance.
Testicular cancer is a rare tumor accounting for only 1% to 2% of all cancers in men. An American Caucasian man has an estimated 0.2% cumulative lifetime risk for developing testicular cancer. Despite the relative rarity of this tumor, it is still the most common solid tumor in young men aged 20 to 35 years and the incidence has increased by 61% from 1973 to 2003, with the majority of the rise caused by seminomas rather than nonseminomas. In 2010, there was an estimated 8480 new diagnoses and 350 deaths from testicular cancer in the United States. Approximately 60% of testicular cancers will have seminoma histology and 80% of these men, or approximately 4300 men, will have disease confined to the testicle (stage I). Thus, stage I seminoma is the most common presentation of testicular cancer.
Adjuvant treatment options for stage I seminoma include surveillance, radiation, and chemotherapy. Historically, adjuvant radiation had been the treatment of choice with excellent cure rates and overall survival rates. Chemotherapy has recently emerged as an alternative option, although longer follow-up is required to ensure that long-term relapse rates and toxicities are acceptable in comparison to radiation. Despite excellent results for both adjuvant chemotherapy and radiotherapy (RT), many concerns have been raised in regards to the potential long-term toxicities, such as secondary cancers, gonadal toxicity, and cardiac toxicity.
To minimize the burden of treatment, there has been a shift away from adjuvant treatments for stage I testicular seminomas toward surveillance protocols for seminoma survivors. This article reviews the evidence for all adjuvant treatment options for stage I testicular seminomas with a particular focus on surveillance.
Initial evaluation and management
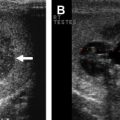
The initial management of a testicular mass or suspected testicular seminoma includes a full history and physical examination. A testicular ultrasound is helpful in differentiating between a solid mass and a potential hydrocele. After confirmation of a solid testicular mass, an inguinal orchidectomy should be performed rather than testicular biopsy. Histology of the testicular mass will guide further adjuvant treatments. Routine laboratory testing includes a complete blood count (CBC); creatinine; and tumor markers, including βHCG, AFP, and LDH. A computed tomography (CT) scan of the abdomen and pelvis and a chest radiograph should be obtained to complete staging. If the CT abdomen and pelvis demonstrates metastatic lymph nodes, a CT scan of the thorax should be included to better evaluate for lung metastases.
Surveillance
There is now mature data demonstrating that patients with stage I seminoma enrolled in surveillance protocols have a relapse rate of 15% to 20% ( Table 1 ). The largest series of patients placed on a surveillance protocol is from Canada with 421 patients with a median follow-up of 8.2 years and a 5-year relapse-free rate of 85.5%. Similarly, the Danish Testicular Cancer Study Group (DATECA) reported on 394 patients with a median follow-up of 60 months and a relapse rate of 17%. Interestingly, 2 independent Japanese studies report lower relapse rates of 10% to 11%, which may represent differences in tumor biology compared with North American and European data. The most common site of relapse was the para-aortic lymph nodes in 82% and 89% of all relapses in the Danish and Canadian series, respectively. Relapses are typically detected at 12 to 18 months in most series; however, late relapses more than 4 years from orchidectomy have been reported. Ultimately, patients managed with surveillance can expect excellent cause-specific survival rates approaching 100%, which is attributable to highly effective salvage radiation or chemotherapy. A review of the Princess Margaret Hospital (PMH) database demonstrated a 6-fold decrease in treatment episodes per patient in patients managed with surveillance (0.16) compared with patients managed with adjuvant RT (1.05).
| Author | Year | Median Follow-up (mo) | Number of Patients | Relapse (Number of Patients) | Relapse (%) | Cause-Specific Survival (%) |
|---|---|---|---|---|---|---|
| Horwich et al | 1992 | 62 | 103 | 17 | 16.5 | 100.0 |
| Ramakrishnan et al | 1992 | 44 | 72 | 13 | 18.0 | 100.0 |
| Von der Maase et al | 1993 | 48 | 261 | 49 | 18.8 | 98.9 |
| Oliver et al | 2001 | 98 | 110 | 21 | 19.0 | 100.0 |
| Germa-Lluch et al | 2002 | 33 | 233 | 38 | 16.0 | 100.0 |
| Daugaard et al | 2003 | 60 | 394 | 69 | 17.5 | 100.0 |
| Warde et al | 2005 | 98 | 421 | 64 | 15.2 | 99.7 |
| Yoshida et al | 2009 | 124 | 64 | 7 | 11.0 | 98.4 |
| Kamba et al | 2010 | 45 | 186 | 19 | 10.0 | 100.0 |
Predictors of Relapse
The identification of tumor and patient characteristics for predicting tumor relapse has evolved over the past decade. The PMH has reported tumor size and patient age as key predictors for relapse, whereas small vessel invasion approached statistical significance. The Royal Marsden Hospital reported vascular or lymphatic invasion as the only significant risk factor. To better identify these risk factors, 638 patients were pooled and analyzed from the databases of the Royal Marsden Hospital, DATECA, PMH, and the Royal London Hospital. On multivariate analysis, only tumor size greater than 4 cm and rete testis invasion were significant predictors for relapse following orchidectomy. A 5-year relapse rate of 12.2% was observed if neither risk factor was present compared with 15.9% and 31.5% if one or both risk factors were present. A validation of this risk-stratification model was performed using 687 patients with stage I seminoma managed with surveillance from the Copenhagen National Hospital, PMH, and British Columbia Cancer Agency. This study found that tumor size remained the only predictor for relapse on univariate analysis, whereas rete testis invasion was no longer statistically significant.
Risk-Adapted Models
Using the prognostic model based on the risk factors (tumor size >4 cm and rete testis invasion) identified earlier, the Spanish Germ Cell Cancer Cooperative Study Group has reported a risk-adapted management approach for stage I seminoma. Patients with no risk factors were considered low-risk and were managed on a surveillance protocol. Patients with a single risk factor had an intermediate risk of relapse, whereas patients with both risk factors were considered to be at high-risk for relapse. Both intermediate and high-risk groups were treated with 2 cycles of adjuvant carboplatin. This study confirmed the findings of the multi-institutional pooled analysis that low-risk patients had a small risk of relapse.
This current risk-adapted approach is problematic because it does not sufficiently identify patients at risk of relapse. Patients in the high-risk group have a predicted 30% relapse rate, which still results in 70% of patients receiving unnecessary treatment. Furthermore, a recent pooled analysis was unable to validate this prognostic model and one of the prognostic factors, rete testis invasion, is no longer a predictor of relapse. The current standard practice at the PMH is to offer surveillance protocol as the management option of choice for all patients with stage I seminoma. Treatment with either radiation or chemotherapy is reserved in the event of a relapse.
Surveillance Schedule
An optimal surveillance schedule has yet to be universally adopted. However, evidence-based recommendations have been published regarding the frequency of follow-up based on the risk of relapse per year. These guidelines suggest increased surveillance with CT scans for the first few years following orchidectomy when the risk of relapse is the highest at 5% per year. Screening becomes less frequent as the risk of relapse decreases until the 10th year when routine screening is discontinued. These recommendations have been adopted by the European Germ Cell Cancer Consensus Group (EGCCCG). The National Comprehensive Cancer Network (NCCN) guidelines are similar with 3 to 4 abdominopelvic CT scans annually for the first 3 years, then every 6 months for the next 4 years and then annually until 10 years of follow-up have been completed. The recommendations adopted by the EGCCCG for the follow-up and investigations for patients managed with surveillance, radiation and carboplatin are included in Tables 2 and 3 .
| Annual Hazard Rate (%) | Frequency | Surveillance | Extended Field RT | Para-Aortic RT | Carboplatin x1 |
|---|---|---|---|---|---|
| >5.0 | 3x/y | First to second y | NA | NA | NA |
| 1.0–5.0 | 2x/y | Third to fourth y | First to third y | First to third y | First to third y |
| 0.3–1.0 | 1x/y | 5th–10th y | Fourth to sixth y | Fourth to sixth y | Limited data |
| <0.3 | Cease | After 10 y | After 6 y | After 6 y |
| Investigation | Surveillance | Extended-Field RT | Para-Aortic RT | Carboplatin x1 |
|---|---|---|---|---|
| CT abdomen | Yes | No | No | Yes |
| CT pelvis | Yes | No | Yes | Yes |
| Chest radiograph | Yes | Yes | Yes | Yes |
CT Screening Radiation Exposure
Despite efforts to diminish potential risks from adjuvant treatments by placing patients on surveillance protocols, concerns remain in regards to the potential toxicities associated with radiation exposure for patients undergoing routine and frequent CT scans. Over the course of 10 years of follow-up, patients on the EGCCCG program would receive 15 CT scans, whereas patients on the NCCN guidelines would receive 21 scans. An average abdominopelvic CT scan exposes patients to approximately 10 to 20 mSV. Over the course of follow-up, patients may receive up to 420 mSV of radiation on a surveillance protocol.
The stochastic effects of radiation, the induction of cancer or a germ line mutation, increase in probability as dose increase. These effects are not considered to have a minimum threshold radiation dose and therefore the risk of carcinogenesis still exists even in individuals who are exposed to low doses of radiation. Data from Japanese atomic bomb survivors have been used to model radiation dose to organs from radiation exposure. This model estimates that 0.6% to 2.0% of all cancers in the United States are attributable to radiation exposure from CT imaging. Although some may view this as an overestimation, this data remains the best available estimate of the carcinogenic risk of radiation exposure from CT scans. Based on retrospective data, the risk of radiation-induced carcinogenesis is thought be higher in younger patients, which is of particular concern in the seminoma patient population who are typically in their third or fourth decade of life at diagnosis.
Current research efforts to reduce radiation exposure in seminoma survivors are underway. In the United Kingdom, the Medical Research Council (MRC) is conducting the TE24 clinical study to evaluate magnetic resonance imaging (MRI) screening as an alternative to conventional CT scans for detection of relapse. The PMH has established an investigational low-dose CT scan protocol, which decreases radiation exposure for each CT scan by approximately 50%. Reduction of radiation exposure comes at the expense of degraded image quality, but in the majority of cases the quality is sufficient to detect relapse. In the future, low-dose CT scans or MRI scans may be incorporated into future surveillance protocols. Elimination of routine imaging of the pelvic lymph at low risk of relapse may lead to a form of targeted imaging where solely the para-aortic lymph nodes are monitored on a regular basis, although this approach remains to be validated. MR lymphography is becoming increasingly used in a variety of different cancer sites to increase the specificity and sensitivity of lymph node metastasis. There may be a role for MR lymphography in the surveillance setting for future patients with seminomas.
Surveillance
There is now mature data demonstrating that patients with stage I seminoma enrolled in surveillance protocols have a relapse rate of 15% to 20% ( Table 1 ). The largest series of patients placed on a surveillance protocol is from Canada with 421 patients with a median follow-up of 8.2 years and a 5-year relapse-free rate of 85.5%. Similarly, the Danish Testicular Cancer Study Group (DATECA) reported on 394 patients with a median follow-up of 60 months and a relapse rate of 17%. Interestingly, 2 independent Japanese studies report lower relapse rates of 10% to 11%, which may represent differences in tumor biology compared with North American and European data. The most common site of relapse was the para-aortic lymph nodes in 82% and 89% of all relapses in the Danish and Canadian series, respectively. Relapses are typically detected at 12 to 18 months in most series; however, late relapses more than 4 years from orchidectomy have been reported. Ultimately, patients managed with surveillance can expect excellent cause-specific survival rates approaching 100%, which is attributable to highly effective salvage radiation or chemotherapy. A review of the Princess Margaret Hospital (PMH) database demonstrated a 6-fold decrease in treatment episodes per patient in patients managed with surveillance (0.16) compared with patients managed with adjuvant RT (1.05).
| Author | Year | Median Follow-up (mo) | Number of Patients | Relapse (Number of Patients) | Relapse (%) | Cause-Specific Survival (%) |
|---|---|---|---|---|---|---|
| Horwich et al | 1992 | 62 | 103 | 17 | 16.5 | 100.0 |
| Ramakrishnan et al | 1992 | 44 | 72 | 13 | 18.0 | 100.0 |
| Von der Maase et al | 1993 | 48 | 261 | 49 | 18.8 | 98.9 |
| Oliver et al | 2001 | 98 | 110 | 21 | 19.0 | 100.0 |
| Germa-Lluch et al | 2002 | 33 | 233 | 38 | 16.0 | 100.0 |
| Daugaard et al | 2003 | 60 | 394 | 69 | 17.5 | 100.0 |
| Warde et al | 2005 | 98 | 421 | 64 | 15.2 | 99.7 |
| Yoshida et al | 2009 | 124 | 64 | 7 | 11.0 | 98.4 |
| Kamba et al | 2010 | 45 | 186 | 19 | 10.0 | 100.0 |
Predictors of Relapse
The identification of tumor and patient characteristics for predicting tumor relapse has evolved over the past decade. The PMH has reported tumor size and patient age as key predictors for relapse, whereas small vessel invasion approached statistical significance. The Royal Marsden Hospital reported vascular or lymphatic invasion as the only significant risk factor. To better identify these risk factors, 638 patients were pooled and analyzed from the databases of the Royal Marsden Hospital, DATECA, PMH, and the Royal London Hospital. On multivariate analysis, only tumor size greater than 4 cm and rete testis invasion were significant predictors for relapse following orchidectomy. A 5-year relapse rate of 12.2% was observed if neither risk factor was present compared with 15.9% and 31.5% if one or both risk factors were present. A validation of this risk-stratification model was performed using 687 patients with stage I seminoma managed with surveillance from the Copenhagen National Hospital, PMH, and British Columbia Cancer Agency. This study found that tumor size remained the only predictor for relapse on univariate analysis, whereas rete testis invasion was no longer statistically significant.
Risk-Adapted Models
Using the prognostic model based on the risk factors (tumor size >4 cm and rete testis invasion) identified earlier, the Spanish Germ Cell Cancer Cooperative Study Group has reported a risk-adapted management approach for stage I seminoma. Patients with no risk factors were considered low-risk and were managed on a surveillance protocol. Patients with a single risk factor had an intermediate risk of relapse, whereas patients with both risk factors were considered to be at high-risk for relapse. Both intermediate and high-risk groups were treated with 2 cycles of adjuvant carboplatin. This study confirmed the findings of the multi-institutional pooled analysis that low-risk patients had a small risk of relapse.
This current risk-adapted approach is problematic because it does not sufficiently identify patients at risk of relapse. Patients in the high-risk group have a predicted 30% relapse rate, which still results in 70% of patients receiving unnecessary treatment. Furthermore, a recent pooled analysis was unable to validate this prognostic model and one of the prognostic factors, rete testis invasion, is no longer a predictor of relapse. The current standard practice at the PMH is to offer surveillance protocol as the management option of choice for all patients with stage I seminoma. Treatment with either radiation or chemotherapy is reserved in the event of a relapse.
Surveillance Schedule
An optimal surveillance schedule has yet to be universally adopted. However, evidence-based recommendations have been published regarding the frequency of follow-up based on the risk of relapse per year. These guidelines suggest increased surveillance with CT scans for the first few years following orchidectomy when the risk of relapse is the highest at 5% per year. Screening becomes less frequent as the risk of relapse decreases until the 10th year when routine screening is discontinued. These recommendations have been adopted by the European Germ Cell Cancer Consensus Group (EGCCCG). The National Comprehensive Cancer Network (NCCN) guidelines are similar with 3 to 4 abdominopelvic CT scans annually for the first 3 years, then every 6 months for the next 4 years and then annually until 10 years of follow-up have been completed. The recommendations adopted by the EGCCCG for the follow-up and investigations for patients managed with surveillance, radiation and carboplatin are included in Tables 2 and 3 .
| Annual Hazard Rate (%) | Frequency | Surveillance | Extended Field RT | Para-Aortic RT | Carboplatin x1 |
|---|---|---|---|---|---|
| >5.0 | 3x/y | First to second y | NA | NA | NA |
| 1.0–5.0 | 2x/y | Third to fourth y | First to third y | First to third y | First to third y |
| 0.3–1.0 | 1x/y | 5th–10th y | Fourth to sixth y | Fourth to sixth y | Limited data |
| <0.3 | Cease | After 10 y | After 6 y | After 6 y |
| Investigation | Surveillance | Extended-Field RT | Para-Aortic RT | Carboplatin x1 |
|---|---|---|---|---|
| CT abdomen | Yes | No | No | Yes |
| CT pelvis | Yes | No | Yes | Yes |
| Chest radiograph | Yes | Yes | Yes | Yes |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree