Management of Spinal Cord and Cauda Equina Compression
Sharon M. Weinstein
Epidemiology
The spine is the most frequent site of bony involvement in patients with malignant metastases (1). The major complications of spinal neoplasm are pain and neurologic injury. Compression of neural structures may be caused directly by the tumor mass or by the displacement of bony fragments into the spinal canal. Tumor of the vertebral bodies has been demonstrated in 25–70% of patients with metastatic cancer (2), and spinal metastases are present in 40% of patients who die from cancer (3). Metastatic lesions from other primary malignancies are three to four times as common as primary bony tumors of the spine (4).
Each year in the United States, approximately 20,000 patients with cancer are treated for malignant epidural compression (EC) of the spinal cord and/or cauda equina. It has been estimated that EC affects 5–10% of adult solid tumor patients and 5% of pediatric solid tumor patients (5, 6). These percentages are corroborated by autopsy series (4, 7).
Half of all patients initially presenting with EC are not known to have cancer at the time that pain or neurologic deficits begin (8). Therefore, it is common for EC to be the presenting symptom of malignancy.
The distribution of spinal tumors reflects the prevalence of primary malignancies as well as the physiology of metastasis. Multiple myeloma is the most common primary bone tumor, representing 10–15% of malignant epidural spinal disease. Osteogenic sarcoma is the second most common primary spinal tumor, usually affecting children and adolescents. Fifty percent of chordomas affect the sacrococcygeal bones and 35% affect the base of the skull. Chondrosarcoma and Ewing’s sarcoma are other bone tumors that may be primary in the vertebrae, although this is rare.
Primary tumors of the breast, lung, and prostate commonly spread to the spinal column. The spine is also a frequent site of metastasis of a nonspinal primary osteogenic sarcoma. Spinal metastases are less common in renal carcinoma, melanoma, soft tissue sarcoma, Ewing’s sarcoma, germ cell tumors, neuroblastoma; and carcinomas of the head and neck, thyroid, and bladder. Rarely, malignant neoplasms of the brain, pancreas, liver, or ovary affect the bony spinal column.
Ten percent of symptomatic spinal metastases originate from unknown primary tumors (3). Some malignancies spread to the intraspinal space without directly affecting the bone. Lymphoma and neuroblastoma often invade the spinal canal through the intervertebral foramina. Ewing’s sarcoma, as well as osteosarcoma, may be primary in the epidural space. Primary epidural tumors are rare.
By location in the spinal column, thoracic metastases are estimated to occur twice as frequently as lumbar metastases and four times as frequently as cervical metastases (2). Almost two thirds of metastatic spinal lesions present clinically in the thoracic region (9), although in some autopsy series, lesions of the lumbar spine have been most prevalent (3). The level of spinal involvement varies with the tumor type. Breast and lung tumor metastases are equally distributed throughout the spine. Prostate, renal, and gastrointestinal metastases are more often found in the lower thoracic, lumbar, and sacral levels. Tumors of the uterus and uterine cervix most commonly spread to the lower lumbar and sacral spine. Pancoast tumors of the apex of the lung extend directly into the cervicothoracic spine in 25% of cases (9), often by intraforaminal extension. Multiple noncontiguous levels of spinal tumor are present in 10–38% of cases (10); this pattern is relatively less common in patients with lung cancer (8).
EC is caused by the direct extension of the tumor from the vertebral body in 85–90% of cases (9). In pediatric patients, EC due to tumor of the posterior elements is more likely, and intraforaminal spread of tumor from paraspinal sites also occurs more frequently than in adults (10). It is noted, however, that tumor metastases in the epidural space seldom breach the dura (3, 11).
The prevalence of EC varies according to the tumor type. In one series of 103 patients with lung cancer, 26% with squamous histology, 9% with adenocarcinoma, and 14% with small cell tumors had spinal cord compression (12). The prevalence of all neurologic complications in this series was approximately 40%.
Breast cancer accounts for almost one fourth of EC diagnosed in cancer hospitals. Vertebral metastases are identified in 60% of patients with breast cancer, and multiple levels of compression are common. EC is rarely the initial presentation or an early finding in breast cancer (13).
Approximately 7% of patients with prostate cancer develop EC. EC was noted in 12.2% of patients with poorly differentiated tumors and 2.9% of those with well-differentiated tumors (14). The average time from initial prostate cancer diagnosis to EC is 2 years, although it is shorter in stage D2. In approximately 30% of prostate cancer patients with EC, it is the initial manifestation of the cancer (15).
Renal cell carcinomas may also cause EC secondary to bony metastasis. Testicular cancer rarely metastasizes to bone but may grow into the spinal canal from the retroperitoneal space. Malignant melanoma may produce EC from vertebral disease,
but intradural and leptomeningeal involvement are probably more common. Head and neck cancers rarely metastasize beyond the cervical lymph nodes; approximately 80% of distant metastases are detected within 2 years of initial diagnosis. Therefore, a patient with head and neck cancer presenting with EC after 2 years should be evaluated for a second primary malignancy. EC occurred at all levels of the spine in one small series of patients with head and neck cancers (16).
but intradural and leptomeningeal involvement are probably more common. Head and neck cancers rarely metastasize beyond the cervical lymph nodes; approximately 80% of distant metastases are detected within 2 years of initial diagnosis. Therefore, a patient with head and neck cancer presenting with EC after 2 years should be evaluated for a second primary malignancy. EC occurred at all levels of the spine in one small series of patients with head and neck cancers (16).
Esophageal cancers may rarely cause EC by direct invasion to the thoracic spinal column (17). Carcinoid tumors are associated with neurologic complications in <20% of cases; the most frequent is EC due to spinal metastases, generally a late complication.
In plasmacytoma and multiple myeloma, EC is usually due to bony collapse, occurring in >10% of patients. Hodgkin’s disease and non-Hodgkin’s lymphomas are associated with a 5% incidence of EC, usually in the presence of extranodal or extensive nodal disease. The thoracic spine is most often involved, in many cases by intraforaminal spread of tumor (18). Patients with EC due to lymphoma are at high risk for meningeal disease. Cerebrospinal fluid (CSF) examination should be considered along with spinal imaging, as concurrent meningeal lymphoma is common and affects the antineoplastic treatment regimen. Vertebral compression fracture with radicular pain is a rare presenting sign of acute leukemia (19).
EC is the presenting sign of cancer in up to 30% of pediatric cases. The time interval to presentation with EC may be twice as long in children without a known cancer, compared to those already diagnosed with malignancy (20). Children without a cancer history presenting with EC are often initially misdiagnosed (6). EC is the most frequent neurologic complication of Ewing’s sarcoma (21).
Differential Diagnosis
The differential diagnosis of back pain and neurologic dysfunction secondary to EC includes benign tumors; it is interesting to note that meningiomas occur frequently in patients with breast cancer (2). Given its high prevalence, coexisting nonmalignant disease of the spine may affect as many as 30% of patients with EC (22). Degenerative, inflammatory, and infectious processes affect the spinal structures (23). Soft tissue injuries causing back pain are very common. Trauma is the most common cause of back pain in children; other nonmalignant conditions such as Scheuermann’s disease and scoliosis (24) also present in this age-group. Back pain in patients with cancer may be a secondary symptom caused by vertebral osteoporosis owing to radiation therapy or corticosteroids.
Spinal cord or cauda equina dysfunction may be related to direct tumor or treatment effects, without EC. Leptomeningeal disease, intradural extramedullary or intramedullary spinal cord disease, paraneoplastic necrotizing myelopathy, and myelopathy induced by radiation or intrathecal chemotherapy should be considered if no epidural compressive lesion is found. Myelopathy is a late complication of radiation; epidural lipomatosis may be caused by corticosteroid therapy. Vascular events of the spinal cord may occur in association with tumor masses.
Pathogenesis of Neurologic Dysfunction and Pain
The high incidence of metastasis to the vertebrae, despite their poor blood supply, is explained by their specific physiologic features. The vertebrae have a large capillary capacity, promoting local stasis of blood. The walls of the vascular sinusoids are discontinuous and intersinusoidal cords form cul-de-sacs for tumor. Tumor products and the products of bone resorption act to stimulate tumor growth (25). Monocytes producing interleukin-1 may promote resorption of normal bone (2). Metastases may occur more commonly in previously damaged bone (26).
Batson’s plexus is a valveless system of epidural veins in which blood may flow rostrally or caudally. On Valsalva’s maneuver, this system drains the viscera and may be a route of metastatic spread. Tumor also reaches the bone through the arteries, lymphatics, and by direct extension.
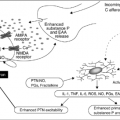
Epidural tumor produces dysfunction of neural structures by direct compression and by secondary demyelination, ischemia, and tissue edema. Inflammation may change vascular permeability and disrupt the blood–spinal barrier at the tumor site. The release of excitatory amino acids by injured neurons further promotes ischemia and injury.
In the initial stage of epidural cord compression, there may be white matter edema and axonal swelling with normal blood flow. These changes are due to direct compression or venous congestion. Over time, progressive compression decreases blood flow and disturbs vascular autoregulation, leading to the development of vasogenic edema. Spinal cord infarction may result from the interruption of venous outflow or occlusion of small arteries or from the interruption of the major arterial supply to the spinal cord (including the artery of Adamkiewicz) or radicular arteries in the intervertebral foramina.
A necrotic cavity, usually located in the ventral portion of the posterior columns or dorsal horn, has been visualized on magnetic resonance imaging (MRI) (10). The effects of cord compression may also be due to coup or contrecoup injury, which is not easily predicted on the basis of the tumor location in relation to the spinal cord. Demyelination as a mechanism of neural dysfunction (5) is supported by pathologic examinations, which demonstrate greater demyelination of white matter than gray matter, a pattern that does not conform to arterial supply. Animal experiments indicate that a more rapid ischemic change produces a greater degree of irreversible neurologic injury (27, 28). Similar observations have been made in the human spinal cord.
Pain due to malignancy of the spine may result from activation of afferent nociceptive neurons by mechanical distortion and inflammatory mediators (nociceptive pain) or from neural dysfunction (neuropathic pain). Nociceptors innervate the periosteum of bone, soft tissues (ligaments and muscles), facet articular cartilage, dura mater, nerve root sheaths, and blood vessels. Vertebral collapse and structural instability can give rise to mechanical pain through injury to these structures, which worsens during spine loading and weight shifting. There may be secondary myofascial pain as well. Neuropathic pain results from altered peripheral and central neural activity that may be induced by injury of the nerve roots, axonal injury, or other processes such as deafferentation (loss of primary sensory input).
Patient Evaluation
Although it is widely recognized that pain is often the first symptom of spinal neoplasm, accurate assessment of back and neck pain in the patient with cancer may be challenging to even the experienced clinician. A complete history and physical examination, including thorough neurologic examination, are essential to localize the underlying pathology. Proper clinical localization is necessary to choose diagnostic and therapeutic interventions correctly (Table 37.1). The importance of obtaining a detailed understanding of the spinal lesion(s) and relationship to symptoms cannot be overemphasized. Inadequate evaluation increases the likelihood of otherwise preventable neurologic compromise. In a retrospective survey of patients with cancer presenting with back pain, misdiagnosis was attributed to poor history, inadequate examination, and insufficient diagnostic evaluation (29). In a review of cancer pain consultations performed by a neurology-based pain service, the comprehensive evaluation of pain led to an identification of new malignant involvement in 65% of cases (30). This underscores the importance of thorough clinical evaluation.
Table 37.1 Patterns of Spinal Tumor Involvement | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
History
Up to 95% of adult and 80% of pediatric patients with EC present with pain (10, 31). The difference in pain prevalence between adults and children may reflect greater difficulty in the pain assessment of and the underreporting of pain in children. Pain may precede other symptoms and signs of EC by 1 year (10). This interval may vary by tumor type; it is generally shorter for lung cancer than breast cancer (32). Overall, patients experience pain for an average of 4–5 months before presentation (3).
Pain may be local at the site of pathology or referred in a nonradicular or radicular (dermatomal) distribution or have combined features. Radicular or root pain is reported in 90% of lumbosacral EC, 79% of cervical, and 55% of thoracic cord compression (32). Radicular pain may be bilateral in thoracic lesions and is often described as a tight band around the chest or abdomen. It is important to note that radicular pain may be experienced in only one part of a dermatome. When a nerve root lesion produces chest or abdominal pain, the complaint may be mistakenly identified as referred pain of visceral origin. Radicular lesions are usually associated with segmental findings on examination. Nonradicular referred pain may be associated with vague paresthesias and tenderness at the painful site. Pain may be continuous at rest and markedly aggravated by body movements (incident pain). Although local pain from a vertebral lesion is worsened with loading due to upright posture, pain due to EC is often greatly increased by lying supine. A lesion confined to the vertebral body may also produce nonradicular referred pain. Disease at C7 may refer pain to the interscapular region, and pain due to disease at L1 may be referred to the iliac crests, hips, or sacroiliac region. Sacral disease often causes midline pain radiating to the buttocks, which is made worse with sitting. Radicular pain, in particular, may be paroxysmal, spontaneous, or provoked by movement or sensory stimulation. Valsalva’s maneuver may produce or aggravate both local and radicular pain. Pain on neck flexion or straight leg raising implies dural traction. Lhermitte’s sign (electric shock–like pain) is a symptom of spinal cord dysfunction. Compression of the cervical spinal cord rarely produces funicular pain, which is pain referred to the lower extremities, thorax, or abdomen as a band of paresthesias. “Pseudoclaudication” of legs may be an isolated lumbar root symptom (2).
The neurologic findings associated with EC also vary. There can be extensive epidural tumor with no neurologic findings on examination. Upper motor neuron weakness may occur with lesions of the spinal cord (above the L1 vertebral body). This finding is present in 75% of patients with EC at diagnosis (9). Sensory changes occur in approximately half of patients at presentation, including paresthesias and sensory loss, which can be segmental or below the level of injury. Sensory complaint without pain is exceedingly rare. Bladder and bowel dysfunction are evident in more than half of patients on presentation with EC; constipation usually precedes urinary retention or incontinence (2).
Examination
The physical examination begins with the observation of posture, spinal curvature, symmetry of paraspinal muscles, extremities, and skin. The practitioner may appreciate tenderness of the spinous processes on palpation or percussion, although this may not correlate with the level of spinal disease. Gibbus deformity and vertebral misalignments are frequently palpable; actual crepitus of the spine is unusual. Tenderness or spasm of the paraspinal muscles may also be noted. Urinary retention may be demonstrated by bladder percussion. Laxity of the anal sphincter may be apparent on digital rectal examination. Specific areas of sacral or coccygeal tenderness may be identified by external palpation, rectal, or pelvic examination.
Spinal maneuvers to elicit pain should be performed carefully. Thoracic and abdominal radicular pain may be provoked on lateral flexion and rotation of the trunk. Increased pain on neck flexion and straight leg raise sign may be “pseudomeningeal” signs of dural traction due to epidural tumor. If neck rigidity is present, the examiner should use extreme caution with range-of-motion maneuvers. Muscle spasm may be triggered by bony instability of the cervical spine, and forced movements may dislodge bony fragments, causing acute spinal cord or brainstem injury.
The neurologic examination reveals positive findings in most patients with EC. The examination should include assessment of mental status, cranial nerves, motor function, reflexes, sensation, coordination, and gait. Proximal lower extremity weakness may be initially evident only as difficulty in rising from a chair. Although weakness due to upper motor neuron dysfunction is usually associated with increased tone and hyperreflexia, acute “spinal shock” can cause a flaccid areflexic paralysis. In the subacute phase of recovery from spinal shock, “mass reflexes” appear consisting of flexor spasms, hyperhydrosis, and piloerection due to autonomic dysfunction. Lower motor neuron weakness may be accompanied by flaccidity,
atrophy, muscle fasciculations, and hyporeflexia. A cervical lesion can produce segmental hyporeflexia in the arm or arms and increased reflexes below. Lesions above the pyramidal decussation of the corticospinal tracts in the lower brainstem may be associated with loss of contralateral abdominal reflexes; lesions below the decussation produce loss of ipsilateral abdominal reflexes. Segmental motor dysfunction due to thoracic nerve root disease may produce asymmetric abdominal muscle contraction and loss of abdominal reflexes. Beevor’s sign (upward movement of the umbilicus on attempted flexion of the trunk) indicates a lesion at or near the T10 level. Lesions of the roots of the upper lumbar plexus produce hip flexion weakness and a dropped knee-jerk reflex; lesions of the roots to the lower lumbar plexus may produce foot drop and diminished ankle-jerk reflex. Loss of bulbocavernosus and anal reflexes may accompany conus and cauda equina lesions (2).
atrophy, muscle fasciculations, and hyporeflexia. A cervical lesion can produce segmental hyporeflexia in the arm or arms and increased reflexes below. Lesions above the pyramidal decussation of the corticospinal tracts in the lower brainstem may be associated with loss of contralateral abdominal reflexes; lesions below the decussation produce loss of ipsilateral abdominal reflexes. Segmental motor dysfunction due to thoracic nerve root disease may produce asymmetric abdominal muscle contraction and loss of abdominal reflexes. Beevor’s sign (upward movement of the umbilicus on attempted flexion of the trunk) indicates a lesion at or near the T10 level. Lesions of the roots of the upper lumbar plexus produce hip flexion weakness and a dropped knee-jerk reflex; lesions of the roots to the lower lumbar plexus may produce foot drop and diminished ankle-jerk reflex. Loss of bulbocavernosus and anal reflexes may accompany conus and cauda equina lesions (2).
Although the sensory examination may help in determining the level of epidural disease, EC results in a broad variation of sensory dysfunction, with incomplete lesions being the rule. The level of reduced sensation may be determined to be up to five segmental levels below, or one to two segments above, the level of cord compression. A sensory level on the trunk sparing the sacral dermatomes may occur in up to 20% of patients with thoracic or high lumbar compression (2). Suspended partial sensory levels or unilateral bands of sensory loss may be seen with spinal cord lesions up to the brainstem. Facial numbness may be due to upper cervical lesions. Lesions of the upper thoracic nerve roots may result in Horner syndrome, with autonomic dysfunction of the face and upper extremity. Compression of the conus of the spinal cord may produce sensory loss in the saddle area (buttocks and perineum) without lower extremity symptoms or signs.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree