Endocrine:
FSH
Estradiol
AMH
Sonographic:
AFC
Previous history:
Age
Endometriosis, pelvic surgery
The woman’s age would be a constant parameter to define a candidate patient for low response. In our centre, we analysed over 5000 in vitro fertilisation (IVF) cycles and observed that the age cut-off point that meant a decrease in the chances of success was 38 years. At this age, a significant fall-off was observed in the pregnancy rate and a rise in low response (Table 4.2). For that reason, we regard a woman as being of advanced age for IVF as of the age of 38.
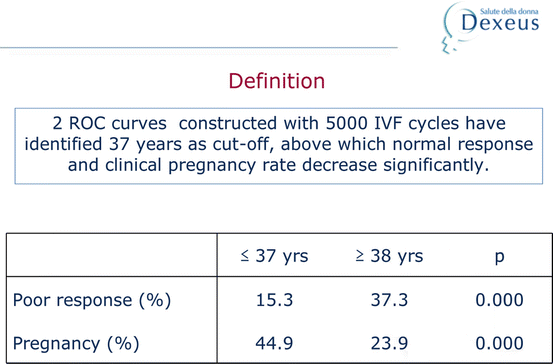
Table 4.2
Old patient

We can say that at this time, the low response markers are those shown in Table 4.2: endocrine (FSH and AMH), ultrasound (antral follicle count (AFC)) and finally, the personal history that may have a bearing (age, endometriosis, ovarian surgery, etc.).
4.2.1 Basal FSH
We regard FSH as a classic marker. High levels of this hormone will indicate a low ovarian follicular reserve and thus a poor response to ovarian stimulation. The use of FSH has its limitations compared with other markers, for example, that it must be done in early follicular phase and presents intercycle variations [6]. It is important to point out the need for determining estradiol at the same time as FSH since normal values for FSH with estradiol levels greater than 80 mg/ml might indicate low ovarian reserve from the negative feedback effect on the central axis.
4.2.2 Anti-Müllerian Hormone
Anti-Müllerian hormone is a glycoprotein that is produced by the granulosa cells of the preantral and antral follicles from 2 to 6 mm in diameter. This hormone indirectly reflects the pool of primordial follicles [7]. Levels of AMH vary with the woman’s age, decreasing as her age increases. One of the problems with AMH is variability depending on the analysis methods that are used [8].
This has advantages over the use of FSH levels. AMH does not have intercycle variations, and it can also be done at any point of the cycle as it remains stable [9]. In 2013, Torner and Seifer [10] published a comparative summary table setting out clearly the differences between the two hormones (FSH and AMH) and ovarian reserve markers.
4.2.3 Antral Follicle Count (AFC)
The antral follicle count is the number of visible follicles (2–10 mm in diameter) during a transvaginal ultrasound performed preferably in early follicular phase (2–5 days of the cycle) [5].
The number of antral follicles correlates with age and with ovarian response. A low number of antral follicles are associated with a poor response to ovarian stimulation in IVF. In our group, lower than seven antral follicles correlates perfectly with a low response to stimulation [11].
In order to assess which marker best predicts ovarian response, we did a retrospective analysis of a total of 863 IVF cycles performed in our centre between 2010 and 2012. We classified the patients as low (<4 oocytes), normal and high responders (>15 oocytes). When the ROC curve of the ovarian response markers was prepared, AMH (<0.4 ng/ml) and AFC (<7 antral follicles) were the two markers that best related to the low response (Fig. 4.1). These results correlate perfectly with those reported by various authors [7–9].
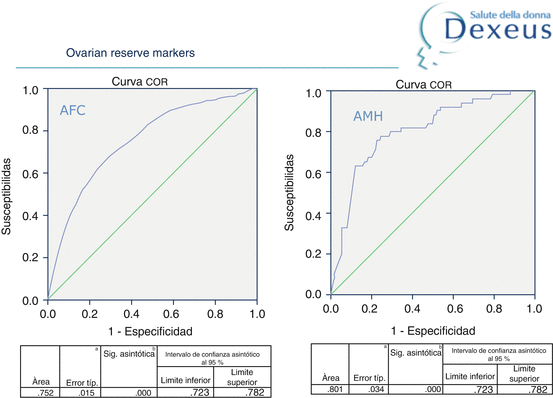

Fig. 4.1
ROC curve of ovarian markers (AFC and AMH)
4.2.4 History of Risk Factors for Low Reserve
In this section, we would like to introduce the concept that every disease that affects the ovary could affect the ovarian reserve and thus reduce the potential for response to ovarian stimulation.
It should be mentioned that the presence of endometriosis can clearly affect ovarian reserve. A review by Somigliana et al. in 2012 [12] clearly shows that patients who undergo ovarian surgery for endometriosis have significantly lower AMH values. Of the 11 studies analysed by the author, 9 state that AMH levels experience a significant reduction after surgery. The scale of this decrease is more obvious when the surgery is bilateral.
4.3 Therapeutic Options in Low Responders
Many treatments have been proposed as solutions for the low responder. From change at the level of the gonadotropin used, increasing the dose or incorporating gonadotropins with LH action, changing the GnRH analogue (agonist in short or ultrashort protocol, GnRH antagonists) and incorporating differing adjuvant treatments such as drugs with androgenic action, growth hormone. Recently, thanks to oocyte vitrification, oocyte accumulation [13] has also been proposed, especially in cases of preimplantation genetic screening [14].
4.3.1 Ovarian Androgenisation
Androgens are products of progesterone metabolism that act as an essential substrate for synthesising estrogens by the granulosa cells (GC), via the two cells-two gonadotropins model: LH binds to the theca cell receptors that stimulate production of androgens. These spread to the granulosa cells (GC) where they are transformed to estrogens, by aromatisation, stimulated by the FSH.
There is proof of the role of the As as positive regulators of follicular development with synergistic effects with FSH in folliculogenesis. The androgens regulate the function of the GC of the small antral follicles by upregulating the expression of FSH receptors (FSH-R). Treatment with testosterone (T) or dihydrotestosterone (DHT) increases FSH-R expression in the GC in monkey and promises the start of the primordial follicle growth. If the aim of impregnation with T is to increase sensitivity to FSH, the treatment must be prior to the start of stimulation with FSH.
In the context of human clinical activity, in women with polycystic ovary syndrome (POS), an increase has been observed in the number of small antral follicles as a result of exposure to high levels of extra-ovarian androgens. The follicles of the women with POS show an increase in expression of FSH receptors (FSH-R) in the GC, high concentrations of A and LH in follicular fluid and a tendency to hyper-response.
Lower FSH-R expression has been shown in the GC of low-response women [15].
Also, it was observed in recent studies [16, 17] that treatment with testosterone for a short period raises sensitivity to FSH and reduces the cancellation and low response rates, as well as the total dose of gonadotropins, in patients with history of low response and normal levels of basal FSH.
A recent meta-analysis [18] shows that the use of transdermal testosterone significantly increases the possibility of clinical pregnancy in patients with history of low response.
These results have been corroborated by various authors. In our group, testosterone was used both in long protocol and in GnRH antagonists in 170 patients with low response, in accordance with the Bologna criteria. The cancellation rate was higher in those with pituitary suppression with GnRH agonists in long protocol compared with the cases in which GnRH antagonists were used. The pregnancy rates per started cycle were similar in both groups (24.3 vs 20.3) (Table 4.3).
Table 4.3
Low responders (Bologna criteria) ovarian androgenisation
GnRH agonists (n = 73) | GnRH antagonists (n = 97) | P value – | |
|---|---|---|---|
Age | 38.9 ± 3.2 | 38.7 ± 3.3 | – |
AMH (ng/ml) | 0.44 ± 0.4 | 0.49 ± 0.5 | – |
AFC | 6.9 ± 3.0 | 6.4 ± 2.8 | – |
Cancellations | 9 (12.3 %) | 2 (2.1 %) | <0.001 |
Days of stimulation | 10.1 ± 1.7 | 10.3 ± 2.1 | – |
Dose of GNS (IU)
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|