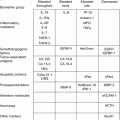
Professional group
Recommendation
US Preventive Services Task Force
Does not recommend routine screening, concluding that “the potential harms outweigh the potential benefits”
American Cancer Society
Does not recommend routine screening; possible screening for women with a family history of ovarian cancer, although “it is not known how helpful” the tests will be in improving survival
American College Of Obstetricians and Gynecologists
Does not recommend routine screening; suggests evaluation of signs and symptoms of ovarian cancer (e.g., pelvic mass, pelvic or abdominal pain, urinary frequency or urgency, increased abdominal size or bloating, or difficulty eating or feeling of fullness)
National Comprehensive Cancer Network
Does not recommend routine screening; recommends screening of high-risk women (i.e., those with either a family history of ovarian or breast cancer or a documented BRCA mutation) with transvaginal ultrasonography and CA-125 measurements every 6 month, starting at the age of 35 years or 5–10 years before the earliest age at diagnosis of ovarian cancer in relatives; recommends strong consideration of risk-reducing prophylactic salpingo-oophorectomy at the completion of childbearing in women with a BRCA mutation
Early Detection of Ovarian Cancer
Early detection is very important to the successful treatment of ovarian cancer. It is estimated that 15 % of ovarian cancer is localized to the ovary, 17 % is regional, and 62 % occurs as distant disease. With tumor spread into the pelvis and upper abdomen, patients often complain of pelvic or abdominal pain or pressure, abdominal swelling, dyspepsia, and early satiety. As the disease progresses, patients note weight loss and increasing pain, and they can develop bowel or urinary tract disorders. Many patients will note a several-month history of vague abdominal discomfort which does not represent marked underlying pathology. A study from the University of Washington [2] noted that ovarian cancer patients’ symptoms occurred 20–30 times per month as compared with two to three times for the clinic population. The severity of symptoms was also significantly higher in cancer patients and of more recent onset. The authors also noted that 44 % of women with ovarian cancer had a triad of bloating, increased abdominal size, and urinary urgency, as compared with only 8 % of clinic controls. They also observed that the important distinction between cases and controls seems to be in the frequency, severity, and duration of the symptoms. Several researchers in the USA and in other countries have found similar results. The same group [2] established a positive symptom index (any of those six symptoms that occurred more than 12 times per month in less than 1 year) and had a sensitivity of 56.7 % for early-stage disease and 79.5 % for advanced disease. More specificity was 90 % for patients older than 50 years and 86.7 % for patients younger than 50 years. Ovarian cancer should be included in the differential diagnosis and testing for the disease should be included in the work-up, if these symptoms are present.
Diagnosis and Staging
Palpation of an adnexal mass during a pelvic examination is considered a diagnostic evaluation for ovarian cancer. Ultrasound examination is a useful noninvasive diagnostic test. Between 13 and 21 % of women undergoing surgery for an adnexal mass will have an ovarian malignancy. Surgical option for management depends on several factors: age, menopausal status, family history, size and complexity of the mass, associated symptoms, CA-125, unilaterality versus bilaterality, and characteristics on ultrasound. Management may include observation with repeat examination, radiographic imaging, and laparoscopy or laparotomy. The preoperative evaluation of a woman with suspected ovarian cancer includes measurement of CA-125, which is elevated in greater than 80 % of patients with advanced EOC. Sensitivity is lower for stage I disease (50 %). CA-125 is highest in serous and lowest in mucinous EOC. In addition, CA-125 is not specific for EOC and found to be elevated in endometriosis, pelvic inflammatory disease, endometrial, and pancreatic cancers. Surgery is necessary for the diagnosis, staging, and treatment of EOC. The bulk of the ovarian tumor could be found on peritoneal surfaces. This peritoneal disease results from shedding of ovarian tumor cells into the peritoneal cavity, circulation of these cells throughout the abdomen and pelvis, and l implantation onto peritoneal surfaces. This is dependent upon the development of sufficient neovasculature to support cell survival and tumor growth. Optimal surgical cytoreduction before the administration of chemotherapy is a common practice in management of ovarian cancer. Optimal cytoreduction could be achieved by reducing the largest residual tumor nodule that remains after debulking surgery to 0.5 cm or less and greater than 1 cm for suboptimal debulking. The goals of initial surgery in ovarian cancer are to diagnose and stage disease and to provide therapeutic benefit with cytoreduction. Accurate histologic diagnosis and staging are required before systemic treatment is established.
Ovarian malignancies are surgically staged according to the FIGO staging system (Table 9.2). Staging laparotomy includes inspection of the peritoneal cavity, including the parabolic gutters, pelvis, and domes of the diaphragm; total abdominal hysterectomy (TAH) and bilateral salpingo-oophorectomy (BSO); liver palpation and biopsy (if indicated); lymph node sampling; omentectomy; and peritoneal washings. The extent of surgical cytoreduction should be fully documented.
Table 9.2
FIGO staging and prognosis of ovarian cancer
FIGO stage | Characteristics | Stage distribution (%) | 10-year survival rate (%) |
|---|---|---|---|
I | Disease confined to the ovaries | ||
IA | One ovary, capsule intact, no ascites | ||
IB | Both ovaries, capsule intact, no ascites | 20 | 73 |
IC | Stage IA or IB plus ascites or washings, capsule ruptures, tumor on ovarian surface | ||
II | Disease confined to the pelvis | 5 | 45 |
III | Disease confined to the abdominal cavity, including surface of the liver; pelvic, inguinal, or para-aortic lymph nodes; omentum or bowel | 58 | 21 |
IIIA | Negative lymph nodes, plus microscopic seeding of peritoneal surface | ||
IIIB | Negative lymph nodes, peritoneal implants <2 | ||
IIIC | Positive lymph nodes and/or abdominal implants >2 cm | ||
IV | Spread to the liver parenchyma, lung, pleura, or other extra-abdominal sites | 17 | <5 |
Treatment Options for Early-Stage Ovarian Cancer
Treatment of early-stage ovarian cancer is dependent on the stage of the disease and extent of surgical cytoreduction. Approximately 25 % of women with ovarian cancer have disease confined to one or both ovaries (FIGO stage I) or to the pelvis (FIGO stage II). Treatment options for stage 1 and stage 2 ovarian epithelial cancers may include:
(A)
Surgery: Total hysterectomy, bilateral salpingo-oophorectomy, partial omentectomy, and surgical staging of the lymph nodes and other tissues in the pelvis and abdomen. Selected premenopausal women in stage I with the lowest-grade tumors in one ovary may be treated salpingo-oophorectomy to preserve fertility.
(B)
Chemotherapy: Patients with stage IA or B disease, grade 1 (or sometimes grade 2) do not need further therapy after surgery. The higher-risk patients (stage IC, stage I/grade 3) are treated with platinum-based chemotherapy to reduce their risk of recurrence.
(C)
Clinical trials with chemotherapy, radiation therapy, or targeted molecular therapy.
Data from the International Collaborative Ovarian Neoplasm trial 1 (ICON1) and Adjuvant Chemotherapy in Ovarian Neoplasm (ACTION) have compared a platinum-containing adjuvant chemotherapy regimen with observation after surgery. They reported over 900 patients with over 4 years of median follow-up; the hazard ratio for recurrence-free survival is 0.64 (95 % confidence interval [95 % CI], 0.50–0.82; P = .001) in favor of adjuvant chemotherapy, and the hazard ratio for overall survival (OS) is 0.67 (95 % CI, 0.50–0.90; P = .008) in favor of adjuvant chemotherapy. A subgroup analysis suggested that the benefits of chemotherapy were in non-optimally staged patients, establishing the clinical importance of surgical staging in early-stage ovarian cancer [3].
The Gynecologic Oncology Group (GOG) studies have shown that patients with stage IA or IB disease (limited to one or both ovaries with no ascites and negative peritoneal washings) and with well- or moderately differentiated histology have a 5-year disease-free survival (DFS) rate of 91 % and a 5-year OS rate of 94 % with surgery alone. This subset of patients is generally not treated with adjuvant therapy. However, it is critical that these patients are fully staged. It has been shown that one-third of apparent early-stage patients will have more advanced stage disease when full staging is done. Also, it was observed that chemotherapy improves progression-free survival (PFS) for patients with stage IA or IB poorly differentiated disease, stage IC, or stage II disease, and these patients should receive adjuvant chemotherapy [4].
GOG in phase 3 study of patients with early-stage ovarian cancer (GOG protocol 157) compared 3 versus 6 cycles of paclitaxel and carboplatin. The 5-year probability of recurrence was 20.1 % for six cycles versus 25.4 % for three cycles, a 24 % reduction in recurrence risk. But the OS was similar for both regimens and the decrease in recurrence was not statistically significant. In addition, analysis of patients from this trial showed that those with high-grade serous histology had a significantly lower risk of recurrence with six compared with three cycles. Based on these findings, it is recommended that a minimum of three cycles of paclitaxel and carboplatin for patients with early-stage disease who are treated with adjuvant chemotherapy and six cycles for those with high-grade serous cancers.
Borderline Ovarian Tumors
Borderline tumors of the ovary are characterized by serous, mucinous or endometrioid histology type, and lack of stromal invasion. The median age at diagnosis is 45, with greater than 34 % of patients being less than 40 years of age. These tumors are managed with TAH and BSO. Twenty-five percent borderline tumors are reclassified as invasive on final review of the histopathology. The surgical management could be limited to unilateral salpingo-oophorectomy with complete surgical staging if patients desire fertility preservation. If the patient has bilateral ovarian tumor, and complete resection can be achieved, ovarian cystectomy is reasonable treatment option.
Complete surgical staging, including exploration of the entire abdominal cavity, peritoneal washings, infrasonic omentectomy, and multiple peritoneal biopsies, is important, as 20 % of patients may have noninvasive as well as invasive metastatic implants (Table 9.3).
Table 9.3
Oncologic and obstetric outcomes in patients with borderline ovarian tumors undergoing fertility-preserving surgery
Author | Patients | Pregnancies | Live births | Recurrences | Deaths |
|---|---|---|---|---|---|
Zanetta et al. [5] | 189 | 44 | N/A | 35 | 0 |
Lim-Tan et al. [6] | 35 | 8 | 6 | 6 | 0 |
Morice et al. [7] | 44 | 17 | 10 | 9 | 0 |
Boran et al. [8] | 62 | 13 | 10 | 4 | 0 |
Fauvet et al. [9] | 162 | 30 | 18 | 27 | 0 |
Donnez et al. [10] | 16 | 12 | 12 | 3 | 0 |
Seracchiolo et al. [11] | 19 | 6 | 6 | 1 | 0 |
Camatte et al. [12] | 17 | 8 | 8 | 9 | 0 |
Morris et al. [13] | 43 | 25 | 16 | 14 | 1 |
Gotlieb et al. [14] | 39 | 22 | 21 | 3 | 0 |
Total | 626 | 185 (50 %) | 107 (58 %) | 111(18 %) | 1 (0.2 %) |
Malignant germ cell tumors account for 5 % of ovarian malignancies, and the median age of affected patients is 19 years. Generally, most of those patients have stage I disease. The management of those patients includes (1) examination and palpation of the ileac and aortocaval nodes with biopsy of abnormal areas intact removal of the tumor, (2) examination and palpation of the omentum with removal of suspicious areas sparing of the fallopian tube if not adherent to the tumor, (3) collection of cytologic washings or harvesting of ascites fluid, and (4) removal of the tumor, (5) sparing the tube and the ovary. It was shown that 90–95 % of malignant germ cell tumors of the ovary are curable with the use of postoperative systemic chemotherapy [20] (Table 9.4).
Table 9.4
The obstetric outcomes in patients with malignant germ cell tumors treated conservatively
Author | Patients | Pregnancies | Live births | Recurrences | Deaths |
|---|---|---|---|---|---|
Kanazawa et al. [15] | 21 | 11 | 9 | 1 | 1 |
Gershenson et al. [16] | 71 | 37 | 30 | 10 | 4 |
Zanetta et al. [17] | 138 | 41 | 28 | 16 | 3 |
Perrin et al. [18] | 45 | 8 | 7 | 4 | 2 |
Tangir et al. [19] | 64 | 47 | 38 | 5 | 3 |
Biopsy of a normal-appearing contralateral ovary is not recommended as this can result in tissue pelvic adhesion and possible mechanical infertility.
Platinum-Based Chemotherapy for Early-Stage Ovarian Cancer
Adjuvant platinum-based chemotherapy has been established as the optimal of care for women with high-risk early-stage EOC [21]. Gynecologic Cancer Intergroup (GCIG) consensus statement [22] and a Cochrane meta-analysis [23] advocated the use of platinum-based chemotherapy. National Comprehensive Cancer Network (NCCN) [24] guidelines recommend three to six cycles of a carboplatin/taxane combination. The GOG-157 trial [25] and other case series [26, 27] show that carboplatin-paclitaxel (CP) is frequently used as the standard of care for early epithelial ovarian cancer. This is due to concerns about suboptimal surgical staging. The GOG definition of comprehensive surgical staging includes total hysterectomy, bilateral salpingo-oophorectomy, resection of all gross disease, aspiration of free peritoneal fluid, peritoneal washings for cytology, infracolic omentectomy, selective bilateral pelvic and aortic node dissections, peritoneal biopsies from four pelvic locations and bilateral paracolic areas, and right diaphragm cytology or biopsy. The ACTION trial [28] found that only 151 (34 %) women were optimally staged. In a study of series of 100 women with apparent early-stage EOC, they underwent a second, comprehensive surgical staging procedure at which 23 were found to have stage III disease [20]. It has been shown that comprehensive surgical staging is critical in all women with early-stage ovarian cancer [29]. It may avoid the need for chemotherapy for some, as subset analysis of the 151 (34 %) optimally staged women in the ACTION trial [28] showed no benefit of adjuvant chemotherapy. The majority of women labeled as having early-stage EOC will have undergone less than comprehensive surgery. Some of these will have occult stage III disease.
It is known that the risk of paclitaxel-related neuropathy increases with cumulative dose [30], and if paclitaxel is used in the initial regimen, the ability to administer sufficient doses to women who relapse will be reduced. This additional toxicity could be justified if there was an overall survival benefit. The ICON three trial [31] compared carboplatin with carboplatin plus paclitaxel. In this trial, 20 % of the population had stage I or II disease. There was no benefit in survival either in the trial as a whole or in the 413 women with early-stage disease.
Published studies (Table 9.5) showed 5-year relapse-free survival (RFS) and 5-year overall survival (OS) in patients with early-stage ovarian cancer treated with systemic chemotherapy following comprehensive surgical staging.
Table 9.5
Published studies of adjuvant chemotherapy on early-stage ovarian cancer
Study | Study type | Stage | Systemic therapy | Women | 5-year RFS | 5-year OS |
|---|---|---|---|---|---|---|
Young et al. [32] | Prospective/randomized | Stage IG3/stage II | Melphalan/32P | 68/73 | 80 %/80 % | 81 %/78 % |
Bolis et al. [33] | Prospective/randomized | Stage IC | Cisplatin/32P | 82/79 | 85 %/65 % | 81 %/79 % |
Trope et al. [34] | Prospective/randomized | Stage I | Carboplatin/observation | 81/81 | 70 %/71 % | 86 %/85 % |
Trimbos et al. [28] | Prospective/randomized | IA–IIA except IA/B G1 | Platinum/observation | 224/224 | 76 %**/68 % | 85 %/78 % |
ICON 1 [3] | Prospective/randomized | Uncertain benefit immediate chemotherapy | Platinum/observation | 241/236 | 73***/62 % | 79 %*/70 % |
Bell [35] | Prospective/randomized | I and II except IA/B G1/2 | CP × 6/CP × 3 | 225/232 | 80 %/75 % | 83 %/81 % |
Bamias et al. [26] | Retrospective/nonrandomized | I–IIB except IA/b G1 | CP × 4 | 69 | 79 % | 87 % |
Shimada et al. [27] | Retrospective/nonrandomized | I–II | CAP × 3 or CP × 3 | 69/31 | 92 % (total group) | |
Platinum-based adjuvant therapy for early-stage EOC; single or combination chemotherapy (Adams [20]) | Retrospective/nonrandomized | IA–IIC | CP × 6/C × 6 | 60/35 | 57 %/54 % | 73 %/62 % |
Adams [20] (Subgroup PS0/1 stage 1) | Retrospective/nonrandomized | IA–IC | CP × 6/C × 6 | 33/20 | 60 %/80 % | 60 %/80 % |
The role of chemotherapy in early-stage epithelial ovarian cancer and the completeness of surgical staging are interlinked. The improved survival of women with suboptimally staged, early-stage ovarian cancer noted in ACTION 2003 is likely to be due to chemotherapy-related treatment of occult disease. The National Institute for Health and Care Excellence (NICE) guidelines (NCCC OC 2011) do not recommend systematic lymphadenectomy but advocate lymph node assessment by palpation and sampling of any suspiciously enlarged nodes. It argues that the morbidity of a comprehensive para-aortic lymphadenectomy cannot be justified. In addition, it has been shown that platinum-taxane combination chemotherapy and platinum-based chemotherapy without taxane were effective in prolonging survival with a significant dose–response relationship among patients with late-stage ovarian cancer. Among those with early-stage tumors, platinum-taxane combination appeared more effective than other chemotherapy regimens [29].
Minimally Invasive Surgery for Patients with Early-Stage Ovarian Cancer
The major deterrents to the general acceptance of laparoscopic staging of EOC have been:
1.
Ovarian cancer represents a major surgical challenge, as it entails more extensive surgery than other gynecological cancers.
2.
Comprehensive surgical staging is of undoubtful importance, as subsequent treatment plans and prognosis will be determined by the stage of disease.
3.
The common and earliest mode of dissemination of ovarian cancer is by exfoliation of cells into the peritoneal cavity.
4.
Last, ovarian neoplasms are often cystic masses and thus are prone to intraoperative rupture.
The real possibility of inadequate staging, port-site recurrence, and intraperitoneal spillage has been counted against the use of minimally invasive surgery for surgical treatment of ovarian cancer.
Laparoscopic Surgery for Ovarian Cancer
Results of published studies addressing laparoscopic staging of EOC are listed in Table 9.6.
Table 9.6
Studies on laparoscopic surgical staging of early-stage ovarian cancer
Study, n | Time in OR, min | EBL, mL | Complications | Conversion | Hospital stay, day | PLN, n | PALN, n | Upstaging, % | Follow-up, month | DFS, % | Overall survival, % |
|---|---|---|---|---|---|---|---|---|---|---|---|
Querleu and Leblanc [36], 9 | 227 | <30 | 0 | 0 | 2.8 | – | 8.6 | 11.1 | NA | NA | NA |
Pomel et al. [37], 10 | 313 | NA | 1 hemoperitoneum, 1 pulmonary embolus | 0 | 4.7 | 7.5 | 8.5 | 10 | NA | 100 | 100 |
Childers et al. [38], 18 | 149/196 | NA | 0 | 0 | 1.6 | – | – | 35.7 | NA | NA | NA |
Tozzi et al. [39], 24 | 176 | NA | 1 chylous ascites, 1 hematoma, 2 lymphocysts | 0 | 7 | 19.4 | 19.6 | 20.8 | 46.4 (2–72) | 91.6 | 100 |
Leblanc et al. [40], 42 | 238 | NA | 1 hematoma, 2 lymphocysts | 0 | 3.1 | 14 | 20 | 19 | 54 (8–116) | 90.5 | 97.6 |
Chi et al. [41], 20 | 321 | 235 | 0 | 0 | 3.1
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|