The medical management of men with castration-resistant prostate cancer (CRPC) has changed dramatically in the last decade. Men can now access several agents developed to extend survival, delay morbidity caused by complications, and preserve quality of life. Strategies to extend survival include docetaxel and cabazitaxel, the CYP-inhibitor abiraterone acetate, the second-generation androgen receptor antagonist enzalutamide, sipuleucel-T immunotherapy, and the α-emitting radionuclide 223 radium. These novel therapies have fostered interest in translational science and a deeper understanding of the underlying biology of CRPC. This article summarizes clinical data and unresolved issues in the use of current and emerging CRPC therapies.
Key points
- •
Agents proven to improve survival for men with castration-resistant prostate cancer include docetaxel and cabazitaxel chemotherapies, androgen/androgen receptor targeting treatments with abiraterone and enzalutamide, sipuleucel-T immunotherapy, and the radionuclide 223 radium.
- •
Signaling through the androgen receptor continues to drive the progression of prostate cancer after development of castration resistance.
- •
Improvements in disease stratification and response assessment are needed to assist patient care and future drug development.
- •
Multiple promising treatments are in the later stages of clinical development.
- •
Participation in clinical trials should be encouraged as part of best standard of care for men with metastatic prostate cancer.
Introduction
The medical management of men with castration-resistant prostate cancer (CRPC) has changed dramatically in the last decade. Multiple successful phase III trials have been reported, and men can now access several agents developed to extend survival, delay morbidity caused by complications, and preserve quality of life. Strategies now proven to extend survival in CRPC include the taxane chemotherapies docetaxel and cabazitaxel, inhibition of androgen biosynthesis with the CYP-inhibitor abiraterone acetate (abiraterone), the second-generation androgen receptor (AR) antagonist enzalutamide, sipuleucel-T immunotherapy, and the α-emitting radionuclide 223 radium. The introduction of these novel therapies has fostered interest in translational science and a deeper understanding of the underlying biology in CRPC. This article summarizes clinical data and unresolved issues in the use of current and emerging CRPC therapies.
Introduction
The medical management of men with castration-resistant prostate cancer (CRPC) has changed dramatically in the last decade. Multiple successful phase III trials have been reported, and men can now access several agents developed to extend survival, delay morbidity caused by complications, and preserve quality of life. Strategies now proven to extend survival in CRPC include the taxane chemotherapies docetaxel and cabazitaxel, inhibition of androgen biosynthesis with the CYP-inhibitor abiraterone acetate (abiraterone), the second-generation androgen receptor (AR) antagonist enzalutamide, sipuleucel-T immunotherapy, and the α-emitting radionuclide 223 radium. The introduction of these novel therapies has fostered interest in translational science and a deeper understanding of the underlying biology in CRPC. This article summarizes clinical data and unresolved issues in the use of current and emerging CRPC therapies.
Overview of drug classes
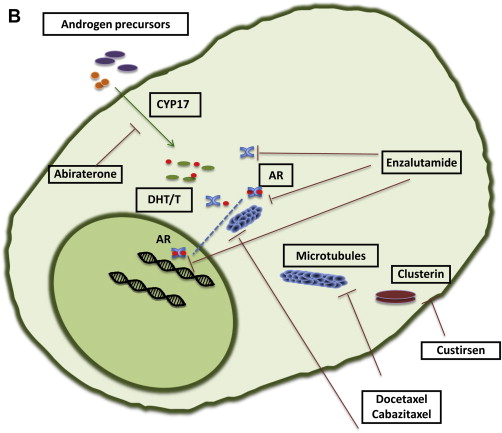
Drug-discovery programs do not always march alongside basic science. Sometimes clinical researchers declare that a compound works before the mechanism by which it is working has been fully elucidated. For this reason, assignment of agents into classes of activity for CRPC may change as our scientific understanding increases ( Fig. 1 ).
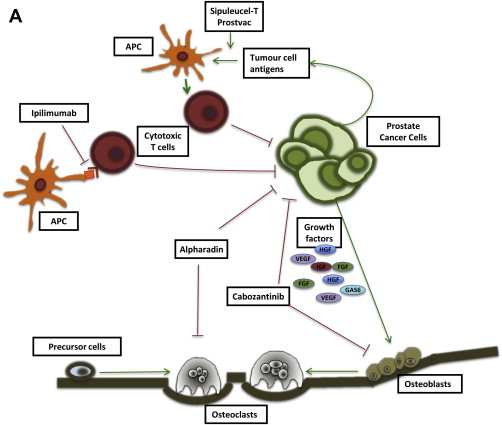
Hormonal therapies remain a mainstay of treatment in advanced prostate cancer, even after development of castration resistance. Ongoing castration by means of bilateral orchiectomy or luteinizing hormone–releasing hormone (LHRH) analogue has invariably been mandated in CRPC trials. In addition, there is compelling evidence to continue systemic castration for abiraterone, as the effect is overcome in the absence of LHRH analogue because of a compensatory LH surge. Docetaxel clearance is reduced in the noncastrate setting, causing potentially greater toxicity than that demonstrated in CRPC trials. Older second-line hormonal therapies such as estrogen preparations and first-generation AR antagonists continue to be used in select CRPC patients, but these strategies lack level III evidence and are not reviewed in this article.
Signaling through the AR remains a key driver of progression in CRPC. Adrenal androgen synthesis accounts for up to 20% of circulating androgens under normal circumstances and, along with intratumoral androgen production, contributes to progression of prostate cancer despite androgen-deprivation therapy. Abiraterone interferes with AR signaling by inhibiting the CYP17 enzyme, blocking androgen production from steroid precursors and thereby reducing androgen levels in the so-called super-castrate range. The second-generation AR antagonist enzalutamide binds to and inhibits AR function without demonstrable agonist activity in the setting of overexpressed or mutated AR.
The taxane chemotherapy agents docetaxel and cabazitaxel have proven utility in CRPC. However, it is no longer clear whether these drugs should be in a separate class as cytotoxics acting as microtubule stabilizers, or whether the activity of taxanes also occurs through inhibition of AR function.
Immune targeting is a current focus of treatment in CRPC, including the specific vaccine therapies sipuleucel-T and Prostvac-VF. There is also considerable interest in the use of agents that cause general modulation of the immune system through blockade of immune checkpoints such as CTLA4 and PD1 to decrease immune tolerance to cancer.
Identifying promising therapies in CRPC has been hampered by the lack of useful intermediate end points and falsely promising signals from phase II trials. Several novel agents are further discussed, including effective targeting of bone metastases with radium-223 ( 223 Ra), the multitargeting MET and vascular endothelial growth factor (VEGF) tyrosine kinase inhibitor cabozantinib, oligonucleotide therapy against the apoptosis protein clusterin, the purported antiangiogenic compound tasquinimod, and targeting of heat-shock proteins (HSPs).
Sequencing and response assessment
Prostate cancer presents with unique disease characteristics that have clinical and research implications. The majority of patients with metastatic disease present with bone metastases, whereas less than 50% have measurable soft-tissue disease. Response assessments therefore depend on a combination of parameters that contain considerable uncertainty such as serial bone and computed tomography scans, prostate-specific antigen (PSA) measurements, and clinical variables such as pain. Overall survival (OS) is considered the gold-standard primary end point for phase III clinical trials, but in the presence of several survival-prolonging treatments it will be increasingly challenging to prove superiority of a novel treatment based on a survival advantage. Intermediate end points or markers of surrogacy for OS are urgently needed. Circulating tumor cells (CTCs) are a promising new technology in patients with advanced prostate cancer. Changes in CTC counts including conversion from unfavorable (CTC ≥5/7.5 mL plasma) to favorable (CTC <5) counts have been associated with longer OS. In the COU-301 abiraterone trial CTC conversion was associated with improved OS as early as 4 weeks after initiation of treatment. For CTCs to meet criteria for surrogacy, further confirmation from phase III clinical trials will be required. CTCs can also be molecularly characterized using AR amplification, phosphatase and tensin homologue (PTEN) and epidermal growth factor receptor (EGFR) assays, and for chromosomal rearrangements (eg, TMPRSS2-ERG rearrangements).
The time between primary diagnosis of prostate cancer and the diagnosis of advanced metastatic disease is generally several years. Collection of fresh tumor tissue later in the disease is therefore important. However, the lack of easily accessible soft-tissue disease and the predominance of bone metastatic disease have made tumor biopsies difficult. Undirected bone marrow biopsies from the iliac crest may be technically feasible in men with prostate cancer, but the published positive yield of 25% was thought to be insufficient to recommend its routine use. Serial bone marrow biopsies in a cohort of patients on abiraterone also proved feasible, but the rate of positive samples was 47% and only 44% of patients had tumor infiltration of 5% or greater.
The availability of several novel treatment options that have different mechanisms of action raises the question of rational combination strategies and sequencing approaches. Combinations of novel agents in clinical trials in advanced prostate cancer are highlighted herein. At present there are no level I data available on sequencing of treatments in advanced prostate cancer, but several case series have been presented at recent oncology conferences. In a published cohort of 35 patients treated with docetaxel following treatment with abiraterone, PSA declines in PSA of at least 50% were reported in 9 patients (26%), with median OS of 12.5 months (95% confidence interval [CI] 10.6–19.4). These data are retrospective and derived from a small set of patients, but seem to suggest a lower activity of docetaxel following abiraterone treatment, which may be explained by shared mechanisms of resistance when targeting the AR, or representing disease more resistant to treatment in general owing to the later stage in the cancer’s evolution.
Targeting of androgen receptor
Abiraterone
Abiraterone (Zytiga; Janssen Pharmaceuticals) is a high-affinity, selective, irreversible inhibitor of cytochrome P450 CYP17, which mediates the conversion of cholesterol to androgen precursors. Abiraterone is structurally related to pregnenolone, a natural substrate of CYP17.
Abiraterone was developed as an acetate prodrug, which increases oral bioavailability. Early clinical trials determined that dosing with a high-fat diet increased drug absorption and exposure.
Following promising early-phase data, abiraterone was compared with placebo in the phase III COU-AA-301 trial. A cohort of 1195 men with progressive CRPC following docetaxel chemotherapy were randomized in a 2:1 ratio to receive abiraterone 1000 mg daily with prednisone 5 mg twice daily, or matched placebo and prednisone. The final analysis after a median follow-up of 20.2 months showed significantly longer survival with abiraterone in comparison with placebo (OS 15.8 vs 11.2 months; hazard ratio [HR] 0.74, 95% CI 0.64–0.86, P <.001). All secondary end points, including time to PSA progression (8.5 vs 6.6 months, HR 0.63; P <.001), radiographic progression-free survival (PFS) (5.6 vs 3.6 months; HR 0.66, P <.001), and PSA response rate (29.5% vs 5.5%; P <.001) favored abiraterone treatment.
The randomized, double-blind, placebo-controlled phase III COU-AA-302 trial evaluated abiraterone in the chemotherapy-naïve setting. A total of 1088 prechemotherapy metastatic CRPC patients were randomized in a 1:1 ratio to receive abiraterone with prednisone or placebo with prednisone. Primary end points for this study were radiographic PFS and OS. The trial was halted following an interim analysis, performed after 43% of the expected OS events. Radiographic PFS was significantly improved with abiraterone (16.5 vs 8.3 months; HR 0.53, 95% CI 0.45–0.62). There was a strong trend in OS (median not reached vs 27.2 months; HR 0.75, 95% CI 0.61–0.93); however, this did not meet the prespecified criteria for statistical significance at interim analysis. No new safety concerns were identified. Abiraterone is currently being tested in the treatment of men with hormone-sensitive prostate cancer as part of the STAMPEDE trial ( NCT00268476 ), as well as in multiple combination studies (see www.clinicaltrials.gov for details).
CYP17 inhibition results in increased corticotropin levels via a negative feedback loop, causing raised levels of upstream adrenal steroids that prevent the development of adrenocortical insufficiency but can cause a syndrome of secondary mineralocorticoid excess. On the COU-AA-301 trial mineralocorticoid-related adverse events were more frequently reported with abiraterone, including fluid retention (31% vs 22%), hypertension (10% vs 8%), and hypokalemia (17% vs 8%), although these were mostly graded as mild to moderate. Mineralocorticoid antagonists (eplerenone) or low-dose glucocorticoids, which decrease corticotropin and steroids upstream of the CYP17 blockade, have been used for the treatment of hypertension or fluid retention secondary to abiraterone, although even these agents can bind and activate mutant AR.
Enzalutamide
Enzalutamide (MDV3100; Xtandi; Astellas Pharma) is a small-molecule AR antagonist that also blocks the AR nuclear translocation and binding to DNA. Unlike bicalutamide, enzalutamide has no known agonist activity in models with AR overexpression. Enzalutamide is administered orally without the need of concomitant steroids.
In a phase I/II study enzalutamide showed significant activity, with PSA declines of 50% or more in 62% of 65 chemotherapy-naïve patients and in 51% of 75 patients pretreated with docetaxel. The main toxicities were fatigue, nausea, and loss of appetite. It was then evaluated in 2 large phase III trials, in the postchemotherapy (AFFIRM) and prechemotherapy (PREVAIL) settings.
The AFFIRM trial randomized 1199 men with CRPC previously treated with chemotherapy to receive enzalutamide 160 mg daily or placebo. The study was unblinded after an interim analysis showed a significant benefit for patients on enzalutamide, with a median OS improvement of 4.8 months (18.4 vs 13.6 months; HR 0.63, 95% CI 0.53–0.75, P <.001). All secondary end points, including PSA response rate (54% vs 2%; P <.001), soft-tissue response (29% vs 4%; P <.001), time to PSA progression (8.3 vs 3 months; P <.001), radiographic PFS (8.3 vs 2.9 months; P <.001) and quality-of-life measures significantly favored the enzalutamide arm.
The main reported adverse events on the enzalutamide arm of AFFIRM were fatigue, diarrhea, and hot flashes. Seizures were reported in 5 patients (0.6%), most of whom had predisposing or concomitant risks. The AFFIRM investigators recommended enzalutamide avoidance in patients with a history of seizure and caution in patients with predisposing risk factors (stroke, alcoholism, brain metastases) or who used medication known to lower the seizure threshold.
The PREVAIL trial ( NCT01212991 ) randomized 1680 asymptomatic or mildly symptomatic chemotherapy-naïve CRPC patients in a 1:1 ratio to enzalutamide or placebo, with primary end points of OS and PFS. Accrual was completed in June 2012, and results are awaited.
Novel Hormonal Therapies
TAK-700
TAK-700 (orteronel; Millennium Pharmaceuticals) is a selective inhibitor of the 17,20-lyase, with less affinity for the 17α-hydroxylase. Although it has a theoretical advantage that it may not require concomitant steroids, current phase III trials mandate the use of concomitant prednisolone. Orteronel showed activity in a phase I/II trial of men with CRPC. PSA declines of at least 50% were achieved in 41% to 63% of the 97-patient expansion cohort, with higher PSA response rates seen with schedules that omitted prednisolone. The safety profile appeared acceptable, with main grade-3 adverse events of fatigue (12%) and hypokalemia (8%). Two large randomized phase III trials are currently evaluating orteronel in the prechemotherapy ( NCT01193244 ) and postchemotherapy ( NCT01193257 ) setting.
VT 464
VT 464 (Viamet) is another selective oral CYP17 inhibitor with selectivity for 17,20-lyase, which potentially avoids the need for concomitant steroids. It is currently undergoing phase I development.
ARN-509
ARN-509 (Janssen Pharmaceuticals) is a small-molecule androgen-signaling inhibitor that, though it has a structure and mechanism of action similar to those of enzalutamide, has been purported to have reduced distribution to the brain in preclinical models. This could be associated with a lower risk of seizures than enzalutamide, allowing higher clinical dose escalation. In preclinical models ARN-509 appeared to have greater efficacy than enzalutamide. A phase I trial of 30 patients with metastatic CRPC showed PSA declines of 50% or more in 43% participants, with fatigue (38%), nausea (29%), and pain (24%) as the most common adverse events of grades 1 to 2. ARN-509 continues to undergo phase I/II testing.
ODM-201
Additional second-generation antiandrogens are in clinical development, including ODM-201 (Orion Pharma), which showed high rates of PSA decline in phase I testing, including in docetaxel-pretreated patients.
TOK-700
TOK-700 (galeterone; Tokai Pharmaceuticals) is a novel, orally available agent that combines CYP17-lyase inhibition with binding and inhibition of the AR and AR degradation. TOK-700 was evaluated in a phase I dose-escalation trial ARMOR1, presented at the 2012 American Association for Cancer Research annual meeting. Treatment was well tolerated, with fatigue, transaminase elevations, and gastrointestinal symptoms as main adverse events. Almost 50% of patients showed a decline in PSA levels of at least 30%, and 22% showed a decline of 50% or greater.
Immunotherapy
Sipuleucel-T
Sipuleucel-T (Provenge; Dendreon Corp) was the first immunotherapy to be approved by the US Food and Drug Administration (FDA) in 2010 for the treatment of prostate cancer. Sipuleucel-T consists of activated antigen-presenting cells (APCs) derived from autologous peripheral blood mononuclear cells. Each APC collection is by a leukapheresis procedure, which usually requires insertion of a “long-line” central venous catheter. Sipuleucel-T is administered as 3 infusions over a 1-month period, and leukapheresis is performed before each of these infusions. The APCs are stimulated ex vivo using a recombinant protein PA2024, which consists of prostatic acid phosphatase fused to granulocyte-macrophage colony-stimulating factor (GM-CSF). Within approximately 3 days of the leukapheresis, the stimulated APCs are reinfused.
In the phase III IMPACT trial, 512 patients with CRPC, bone or lymph node metastases, and a chemotherapy-free interval of at least 3 months were randomized in a 2:1 ratio to sipuleucel-T or placebo treatment. The trial reported an OS benefit of 4.1 months for sipuleucel-T (25.8 vs 21.7 months; HR 0.78, 95% CI 0.61–0.98, P = .03). Other efficacy measures, such as PFS and PSA decline of at least 50%, were equal in both treatment arms. Although prior chemotherapy treatment was not excluded, it is noteworthy that more than 80% of patients were chemotherapy-naïve. In addition, the patients’ characteristics indicate a highly selected population, with Gleason score of 7 or less in 75% patients, while 43% of patients had low-volume bone-only metastases and 52% were free of pain at study entry. The most common sipuleucel-T related adverse events were influenza-like symptoms, including chills (51%), fever (23%), fatigue (16%), nausea (14%) and headaches (11%), mostly of mild to moderate grade, which occurred within 24 hours of infusion and generally resolved within 48 hours.
A smaller randomized trial of 127 patients failed to meet the primary end point of time to tumor progression but reported a survival benefit similar to that of the IMPACT trial (25.9 vs 21.4 months; HR 1.70 for placebo, 95% CI 1.13–2.56), as did the integrated analysis of the D9901 and D9902A randomized phase II clinical trials. A randomized phase II clinical trial, in which patients with detectable serum PSA 3 to 4 months following radical prostatectomy were randomized in a 2:1 ratio between sipuleucel-T or placebo, showed no difference in the primary end point of time to biochemical progression (18.0 vs 15.4 months; HR = 0.936, P = .737). The lack of predictive biomarkers, the high cost of treatment, and the complexity of treatment preparation and administration may limit the use of this treatment. Ongoing phase II clinical trials of sipuleucel-T will investigate the coadministration of immunomodulatory agents such as cyclophosphamide ( NCT01420965 ) or the immune response regulator indoximod ( NCT01560923 ), and also investigate concurrent versus sequential administration of abiraterone ( NCT01487863 ).
Prostvac
Prostvac (Bavarian Nordic) is a recombinant vaccinia-viral expression cassette expressing PSA and costimulatory molecules (B7.1, ICAM-1, and Lfa-3), which is administered subcutaneously. A phase II clinical trial included 125 chemotherapy-naïve patients without visceral metastases and a Gleason score of 7 or less, who were randomized 2:1 to Prostvac plus GM-CSF or placebo. Although PFS was similar in both arms, the median OS was significantly longer with Prostvac than with placebo (25.1 vs 16.6 months; HR 0.56, 95% CI 0.37–0.85). A large 3-arm phase III clinical trial will randomize chemotherapy-naïve men with asymptomatic or minimally symptomatic CRPC between Prostvac ± GM-CSF or placebo ( NCT01322490 ).
Ipilimumab
Ipilimumab (Yervoy; Bristol-Myers Squibb) is a monoclonal antibody against the cytotoxic T-lymphocyte antigen CTLA-4. Activation of T cells by APC requires interaction between the major histocompatibility complex and the T-cell receptor plus simultaneous interaction with a costimulatory molecule (CD28) on T cells. CTLA-4 (CD152) is a T-cell membranous protein and CD28 homologue, which acts as an inhibitor of T-cell activation. Phase I clinical trial data confirmed the safety and tolerability of ipilimumab 3 mg/kg in patients with advanced prostate cancer, and showed 50% or greater declines in PSA in 2 of 14 patients. A phase I clinical trial combining ipilimumab 10 mg/kg with a poxviral-based vaccine targeting PSA and T-cell costimulatory molecule expression proved the feasibility of the combination approach, and reported PSA declines of at least 50% in 6 of 30 patients. Phase III trials of ipilimumab 10 mg/kg versus placebo have been conducted in asymptomatic or minimally symptomatic CRPC patients ( NCT01057810 ) and in symptomatic patients following palliative radiotherapy ( NCT00861614 ), and several phase II clinical trials of ipilimumab in combination with androgen deprivation ( NCT01498978 ; NCT01377389 ) or abiraterone ( NCT01688492 ) are ongoing.
Targeting the interaction between the programmed death 1 (PD-1) receptor on cytotoxic T cells and its ligand PD-L1 has recently been shown to induce prolonged stabilization of disease and some durable tumor regressions in advanced solid tumors. A 3-arm randomized phase II clinical trial of sipuleucel-T, CT-011 (a PD-1 inhibitor), and cyclophosphamide in advanced prostate cancer is ongoing ( NCT01420965 ).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree




