Pre-therapeutic Imaging Evaluation
Adequate imaging is essential for patients with suspected CLM for diagnosis, staging, treatment planning, and evaluation of response to chemotherapy. The choice of imaging technique for pretreatment assessment of CLM depends on the local expertise and availability of imaging modalities. However, computed tomography (CT) and magnetic resonance imaging (MRI) are the most common modalities utilized for diagnosing and evaluating patients with CLM.
Niekel et al. [9] reviewed 39 articles (3,391 patients) and showed that the estimated sensitivities on a per-lesion basis for CT, MRI, and 18F-fluorodeoxyglucose positron emission topography (18F-FDG-PET) were 74.4 %, 80.3 %, and 81.4 %, respectively. Per-patient sensitivities were 83.6 %, 88.2 %, and 94.1 %, respectively. MRI combining gadolinium ethoxybenzyl diethylenetriamine pentaacetic acid (Gd-EOB-DTPA) delayed images and diffusion-weighted imaging has the best performance characteristics for detecting and characterizing liver lesions, particularly those smaller than 10 mm in size [10]. In addition, the usefulness of 18F-FDG-PET has been reported especially for detecting extrahepatic metastases or local recurrence [11, 12]. However, increased sensitivity is usually associated with reduced specificity. Also, limitations of these new imaging modalities include limited availability, high cost, limited access to specialized techniques, and lack of expertise to interpret the results. Therefore, from a practical clinical perspective, CT still plays a central role in characterizing CLM because of its accessibility, practicality, low cost, and acceptable sensitivity/specificity to characterize CLM. At MD Anderson Cancer Center, a CT of chest, abdomen, and pelvis is routinely performed for evaluating patients with CLM [13]. PET and MRI are selectively used.
Evaluation of Resectability
After confirming the patient’s physical status to tolerate surgery and determining his/her tumor distributions, the eligibility for resection in patients with CLM is determined by two factors: oncological benefit and technical feasibility.
Oncological Resectability
From an oncological standpoint, complete resection of all viable disease in patients with CLM is crucial if the patient is to derive the most benefit from surgery. Selection of surgical candidate depends on the presence or absence of extrahepatic disease and tumor response to chemotherapy.
Because lack of extrahepatic disease is associated with the ability to perform curative surgery, careful preoperative screening is important to make this determination. Lung, abdominal lymph nodes, and peritoneum represent the most common sites of extrahepatic disease. However, location of extrahepatic disease plays less of a role in determining outcome so long as complete resection is feasible [14]. In appropriately selected patients, the presence of extrahepatic disease does not necessarily represent an absolute contraindication for surgery, since there are reports of relatively favorable long-term survivals in those who had extrahepatic metastasectomy [15]. Isolated lung metastases or periportal adenopathy has reportedly been associated with a high 5-year survival rate (30–40 %) when complete resection is feasible [16]. Localized peritoneal disease correlates with intermediate 5-year survival rates (15–30 %), whereas para-aortic adenopathy or evidence of multiple sites of extrahepatic disease is rarely associated with good survival after resection of CLM (5-year survivals <15 %) [17]. These data suggest that patients harboring limited extrahepatic disease are amenable to surgical resection with a reasonable expectation for long-term control with adjuvant therapies [18]. When the extrahepatic disease burden is unresectable or uncontrollable, hepatic resection for CLM is contraindicated.
Another important factor in determining resectability is response to chemotherapy. When patients are treated with preoperative systemic therapy, biologic behavior of the tumor can be assessed during treatment. With modern effective chemotherapy, disease progression during preoperative systemic therapy is relatively rare. However, there are patients who occasionally (5–15 %) do have disease progression during receipt of systemic therapy, and development of new lesions is associated with a poor prognosis after CLM resection [19]. In contrast, growth of preexisting intrahepatic lesion itself does not seem to be associated with poor outcomes as long as new lesions do not develop during treatment. Therefore, patients who show this pattern of progression who have resectable lesions should remain candidates for a hepatectomy.
A recent study reviewing LiverMetSurvey international registry reported that although tumor progression during chemotherapy is a negative prognostic factor, surgical resection might still be a viable option with acceptable long-term outcomes. Exceptions include patients with >3 liver metastases, liver tumor size ≥50 mm, and/or serum carcinoembryonic antigen (CEA) level ≥200 ng/mL; in such situations, further chemotherapy is recommended [20].
Technical Resectability
Technical resectability is based on adequate knowledge of liver anatomy, histopathology, and hepatic function, all of which are best evaluated in a multidisciplinary setting with inputs from hepatobiliary surgeons, radiologists, hepatologists, and pathologists. Conventionally, technical resectability has been defined as removal of all viable tumors with a negative margin, leaving behind a minimum of two contiguous segments of hepatic parenchyma that have adequate vascular inflow and outflow and adequate biliary drainage [21]. More recently, the selection of patients with resectable CLM has greatly improved from the enhanced ability to predict future liver remnant (FLR) volume and liver function.
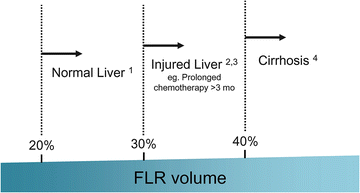
Currently, functional reserve of the liver is estimated by both static and dynamic measurements. The most reliable static variable is the FLR volume. Because absolute volume of FLR against standardized liver volume (SLV) (i.e., sFLR: standardized FLR) has strong correlation with the rates of postoperative morbidity and mortality (Fig. 19.2) [22, 23], minimal requirements of sFLR have currently been set at >20 % in normal liver, >30 % in damaged liver after extensive treatment, and >40 % in cirrhotic liver (Fig. 19.3) [22, 24–27]. In a recent analysis on the clinical impact of duration of systemic therapy and the minimal requirement of sFLR in patients undergoing preoperative chemotherapy, those who underwent more than 3 months of systemic therapy required at least 30 % of sFLR to prevent postoperative hepatic insufficiency [27]. These cutoff values offer a good practical decision making metric in patients requiring major hepatectomy.
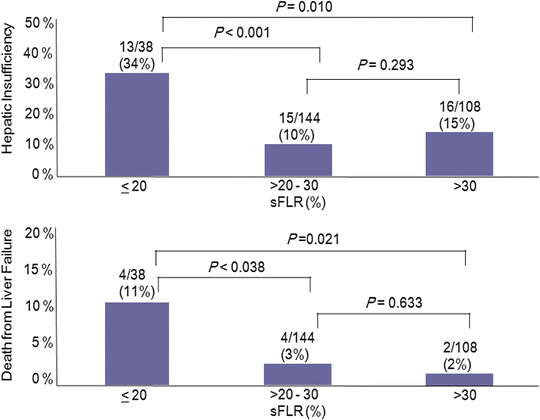

Fig. 19.2
FLR volume and surgical outcomes (Adapted from Ref. [22]. With permission from Wolters Kluwer Health)
In addition to the FLR volume, dynamic measurements such as degree of hypertrophy [23] and kinetic growth rate (KGR) [28] after portal vein embolization (PVE) have also been reported to be sensitive predictors of functional liver reserve in patients undergoing extended hepatectomy. The KGR, defined as the degree of hypertrophy divided by the number of weeks elapsed after portal vein embolization, well predicts underlying liver function and short-term surgical outcomes independent of sFLR or the timing of initial volume assessment. KGR of at least 2 % per week reduces hepatic complications and liver failure-related deaths [28].
Furthermore, indocyanine green clearance test [29] or hepatic scintigraphy [30] has also been reported to be a good indicator of hepatic functional reserve with regard to metabolic function. Because FLR volume itself is not correlated with functional reserve, dynamic measurements should be integrated to estimate the total functional reserve of FLR in individual patients.
Strategies to Increase Resectability
Portal Vein Embolization (PVE)
PVE is a safe, minimally invasive procedure that leads to atrophy of the liver to be resected and compensatory hypertrophy of FLR [31–33]. Based on the baseline status of liver parenchyma, PVE should be considered if a patient is found to have insufficient volume in the pretreatment measurement of FLR. To maximize the regeneration of FLR in PVE, optimal selection of embolic materials [34] and concurrent embolization of segment IV portal vein [35, 36] have been recommended, the latter often has evidence of disease. Our previous work comparing right PVE with and without segment IV embolization revealed significant difference in volume increase rates in segment II + III (median, 26 % vs. 54 %; p = 0.021).
Two-Stage Liver Resection
With limited liver tumor burden including small tumors and anatomically favorably positioned bilateral metastases, a one-stage strategy involving one or more simultaneous partial to lobar hepatic resection is safe and effective [37–42]. In contrast, when extensive bilobar metastases are present (i.e., extensive right lobe disease including disease in segment 4 and one or more lesions in the left lateral segment and/caudate lobe), different surgical strategies are required.
Two-stage liver resection (TSR) is indicated in patients with advanced bilateral CLM who responded to chemotherapy and in whom limited resection can clear the less affected side of the liver before a planned extended contralateral liver resection. In the majority of cases, patients undergo first-stage limited resection of metastases of the left lobe, followed by right PVE with segment IV embolization to allow hypertrophy of the FLR (i.e., left lateral segment and segment 1), and extended right hepatectomy is completed after sFLR meets the volume criteria. A previous study from MD Anderson Cancer Center reported that 72.3 % (47/65) of the patients among planned TSR completed TSR, and the 5-year survival in these patients was 51 % compared to 15 % in the cases treated with chemotherapy only [44].
Associating Liver Partition and Portal Vein Ligation for Staged Hepatectomy (ALPPS) Procedure
Recently, a European group reported safety and efficacy data for a short-interval (median waiting period of 9 days) two-stage liver surgery technique consisting of an initial open right portal vein ligation with in situ splitting of the liver parenchyma followed by re-exploration for right trisectionectomy, named Associating Liver Partition and Portal Vein Ligation for Staged hepatectomy or “ALPPS” [45]. Wedge resection of the left lobe is generally performed at the initial operation so as to render the left lobe disease-free. The combination of portal vein ligation and in situ splitting of the liver to prevent cross-portal circulation between the lobes of the liver was believed to lead to a profound hypertrophy of the FLR. However, preliminary data suggested a high incidence of major morbidity (40 %) and inpatient mortality (12 %) associated with this new procedure.
In our recent study comparing the ALPPS and PVE for patients with very small FLR volume, we demonstrated that right PVE with segment IV embolization may offer equivalent hypertrophy of FLR (62 % vs. 74 %) but with less perioperative bile leak (5.8 % vs. 24 %) and sepsis (0 % vs. 20 %) compared to the ALPPS procedure [46]. Although the time duration from the hemodynamic modulation to surgery was significantly longer in the PVE group (34 days vs. 9 days), this waiting period is oncologically meaningful because it allows selection of patients who would truly benefit from surgical resection while avoiding unnecessary resection of those with disease progression.
Strategies for Synchronous Metastases
Nearly 25 % of patients with colorectal cancer have CLM at the same time the primary tumor is diagnosed (synchronous presentation). The major problem in these patients is that both colectomy and hepatectomy are needed to resect all tumor burdens, either by a simultaneous or in a stepwise fashion. The traditional surgical strategy for patients with resectable synchronous CLM includes resection of the primary tumor followed by chemotherapy and then liver resection (classic strategy). A combined strategy that includes simultaneous resection of the primary colorectal lesion and the liver metastases has also been used to avoid delaying surgical resection of metastatic lesions. However, the limitation with the combined strategy is the associated increased risk of postoperative complications.
With recent advancements in effective chemotherapy, a reverse strategy, in which preoperative chemotherapy is followed by resection of the CLM and then by resection of the colorectal primary at a second operation, has been proposed especially for patients with advanced synchronous CLM. A study comparing these three approaches (classic, combined, and reverse approaches) demonstrated similar surgical outcomes among the three approaches despite patients undergoing the reverse strategy having more extensive disease. Therefore, a reverse strategy should be considered as an alternative approach for treating advanced CLM in patients with synchronous liver metastases and an asymptomatic primary tumor (i.e., no evidence of obstruction, bleeding, intractable pain, or perforation) [47].
Surgical Outcomes
Short-Term Outcomes
A systematic review of short-term results of liver resection reported a mortality rate of 0 % to 6.6 % (median 2.8 %) [48]. The main cause of mortality related to liver resection was hepatic failure (18.4 %), followed by hemorrhage (17.5 %) and sepsis (16.5 %). However, the definition of liver failure varied among institutions, making it difficult to compare surgical risk according to individual criteria for FLR volumes.
Mullen et al. have reviewed 1,059 noncirrhotic patients who underwent major hepatectomy at 3 hepatobiliary centers and found that peak serum bilirubin level of > 7.0 mg/dL was a potent predictor of any (odds ratio [OR] 83.3) or major complication (OR 10.0), 90-day mortality (OR 10.8), and 90-day liver-related mortality (OR 250). Importantly, combining INR with bilirubin did not improve the high sensitivity (93 %) and high specificity (94 %) of bilirubin alone in the predicting liver failure. Therefore, peak bilirubin level of >7.0 mg/dL is defined as “postoperative hepatic insufficiency” and is a potent predictor of “death from liver failure” [49].
Based on the clear definition of hepatic insufficiency, minimal requirement of FLR volume could be analyzed and determined according to the histopathologic status of underlying liver [22, 27], This has also contributed to develop further advance the concept of dynamic measurement of liver volumes such as degree of hypertrophy [23] or kinetic growth rate [28] after PVE as mentioned in the previous section.
The reported overall complication rates after hepatectomy range from 16 % to 44 %. Factors associated with the risk for postoperative complications include complexity of liver resection (number of liver segments to be resected, whether or not a biliary-enteric anastomosis is performed, the need for vascular resection, etc.), intraoperative blood loss and blood transfusion, concomitant major extrahepatic procedure, and patient medical conditions [50]. In a recent study, we compared short-term outcomes of 2,628 liver resections at MD Anderson Cancer Center in two different periods (before and after 2006) and found that overall morbidity rates, hepatic insufficiency, and 90-day mortality have not changed over time, even though the complexity of surgery such as extended hepatectomy, repeated resection, two-stage surgery, or use of preoperative PVE has increased. However, the rate of bile leak has increased over time (3.7 vs. 5.9 % before and after 2006, respectively) which is likely related to the increasing complexity of liver resection. With the systematic use of a new air leak test to detect bile leak, the rate of biliary fistula has significantly decreased over the recent years [51, 52].
Long-Term Outcomes and Prognostic Factors
With the development of effective chemotherapy and strategies for surgical management as mentioned previously, recent series reported the 5-year survival rate after curative resection of CLM to be as high as 58 % [4–6]. However, there is considerable heterogeneity in oncological feature of the tumor and patients and variable degree of aggressiveness of CLM among patients, which lead to a variable 5-year survival rates reported in the literature
Traditionally, large liver tumor size and number of tumor, evidence of bilobar distribution, short disease-free interval, and high serum CEA level before hepatectomy have been regarded as important poor prognostic factors following resection of CLM [38, 39, 53]. However, increasing evidence has suggested that these traditional prognostic factors are losing their clinical significance in the era of effective chemotherapy and increasing use of biologic agents. In the era of effective preoperative chemotherapy, several new criteria have been proposed that appeared to be sensitive in predicting patient survival.
Pathologic Response
Pathologic response to preoperative chemotherapy is a strong predictor of survival outcomes in patients undergoing hepatic resection after preoperative chemotherapy (Fig. 19.4) [54, 55]. Pathologic response is excellent in stratifying both overall and recurrence-free survival of patients who undergo hepatic resection of CLM. However, the limitation in the clinical setting is that pathologic response is difficult to assess prior to surgery.
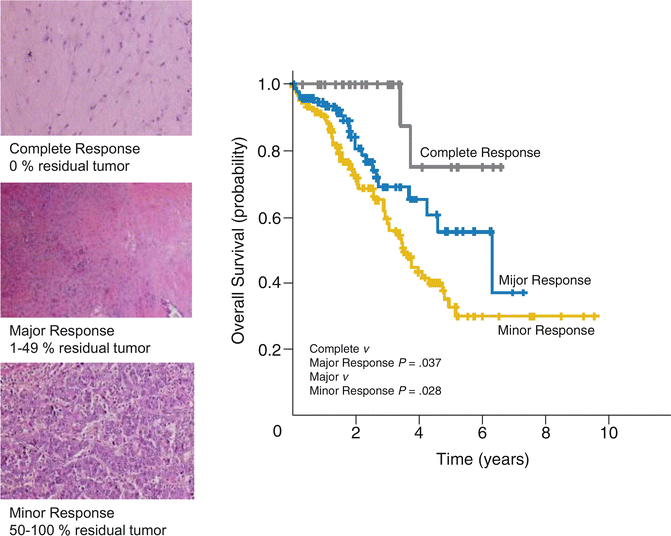

Fig. 19.4
Pathologic response to preoperative chemotherapy (Adapted from Ref. [54]. With permission from American Society of Clinical Oncology)
Radiologic Response
Radiologic response to chemotherapy was conventionally assessed by changes in tumor size according to the Response Evaluation Criteria in Solid Tumors (RECIST) [56–58]. However, recent studies have reported that the RECIST criteria may underestimate the response to chemotherapy since the traditional size-based response criteria can be unreliable [59–62]. To overcome this issue, our group first reported that changes consisting of a “cystic-like” alteration in the texture of tumor seen on CT image (morphologic response) is a better alternative criterion for evaluating response to preoperative therapy in patients with CLM (Table 19.1 and Fig. 19.5) [63, 64]. In a recent validation study with 209 patients, we confirmed that these non-size-based observations were also applicable for patients who were not given bevacizumab. Morphologic response was well correlated with pathologic response, and suboptimal morphologic response was a strong “preoperative” prognostic factors for both overall survival (Hazard ratio [HR] 2.1, 95 % CI 1.2–3.8) and recurrence-free survival (HR 1.8, 95 % CI 1.2–2.8) [64].
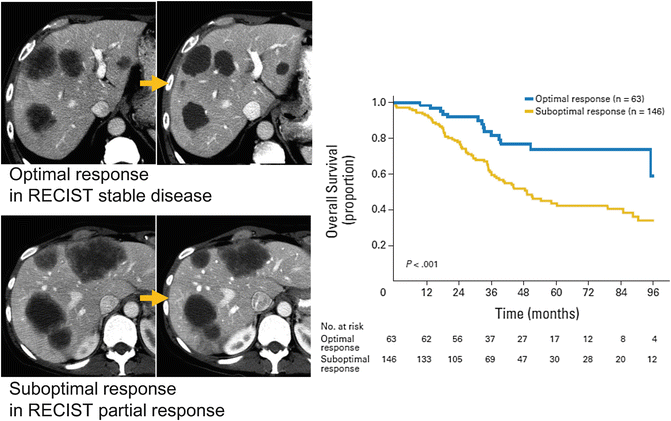

Fig. 19.5
Morphological response to preoperative chemotherapy (Adapted from Ref. [64]. With permission from American Society of Clinical Oncology)
Table 19.1
Definition of CT morphologic groups
Group | Overall attenuation | Tumor-liver interface | Peripheral rim of enhancement |
|---|---|---|---|
3 | Heterogeneous | Ill defined | May be present |
2 | Mixed | Variable | If initially present, partially resolved |
1 | Homogeneous and hypoattenuating | Sharp | If initially present, completely resolved |
Somatic Mutational Status
The variability of the individual CLM in clinical presentation, degree of aggressiveness, and patterns of treatment failure suggests the presence of variability in genotypes and phenotypes among the individual patients. Over the past decades, numerous biomarkers and molecular pathways have been investigated to explain such biologic heterogeneity. Among the molecular candidates, RAS mutation is the most important marker that predicts efficacy of anti-EGFR (epidermal growth factor receptor) biologic agents. Recent studies have clarified that RAS mutation status in clinical practice is likely to expand beyond its current role just as a predictor of response to anti-EGFR agents.
First, RAS mutations independently predict worse overall and disease-free survival after resection of CLM [65–67]. Second, RAS mutation status is also predictive of patterns of recurrence or metastases to other organs. Tie et al. reported higher KRAS mutation rates in lung (62 %), and brain (56 %) colorectal metastases than in primary colorectal cancer (35 %) [68]. Our group also confirmed that patients with RAS mutation undergoing resection of CLM had a worse lung recurrence-free survival than patients with RAS wild type [67]. In another study, RAS mutational status also predicted radiologic and pathologic response in patients treated with preoperative chemotherapy for CLM (Fig. 19.6) [69]. Though the clinical significance of mutation in RAS has not been fully understood, it may offer clinicians the ability to predict outcome at presentation before response to chemotherapy and can serve as a basis for personalized medicine in the near future.
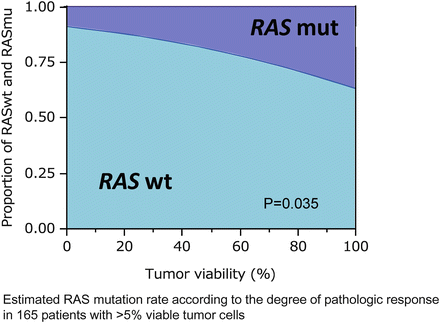

Fig. 19.6
RAS mutations and pathologic response (Adapted from Ref. [69]. With permission from Annals of Surgical Oncology)