Fig. 1
Initial setup for parathyroidectomy—sublpatysmal plane having been developed, the skin edges are protected under edges of a surgical towel kept in place by a simple spring retractor
The sternothyroid muscle is then elevated off of the sternohyoid, in a relatively avascular plane requiring minimal or no electrocautery. When the plane between the strap muscles is difficult to identify, it is helpful to elevate the medial border of the sternohyoid muscle while retracting the thyroid isthmus medially with a Kittner dissector. This accentuates the groove between the two strap muscles and facilitates their separation, which should proceed laterally until the internal jugular vein is identified. In cases where nerve monitoring is used, it is recommended to stimulate the vagus nerve at this stage of the operation to ensure the baseline functional integrity of the nerve monitoring circuit and of the nerve itself [14]. The vagus is identified deep, between the jugular vein and common carotid artery.
Intraoperative parathyroid hormone (IOPTH) monitoring is required to achieve the lowest risk of persistent or recurrent HPT after minimally invasive parathyroidectomy . We obtain the samples by direct venipuncture of the jugular vein using a 22-gauge needle (Fig. 2). Usually, this venipuncture site does not require suture repair if direct pressure is applied for a short time. Other surgeons prefer to have the levels drawn from a large-bore indwelling intravenous (IV) line or an arterial line. However the sample is obtained, care should be taken to avoid hemolysis from vigorous negative pressure or during transfer or handling of the sample, as this results in a substantial artificial decrease in the PTH value [15, 16].
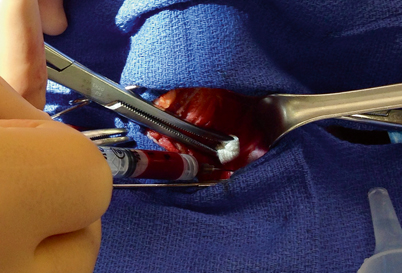

Fig. 2
With sternohyoid muscle retracted laterally (to left), and sternothyroid muscle and thyroid gland retracted medially under a Kitner dissector, PTH level is drawn from the left internal jugular vein
Next, the sternothyroid muscle, which does not extend to the midline, is elevated off of the thyroid gland. The thyroid gland is retracted over the anterior surface of the trachea while dissecting the sternothyroid muscle off of the thyroid capsule while an assistant retracts the strap muscles laterally (Fig. 3). Often, careful and complete mobilization of the sternothyroid muscle is all that is required to identify the parathyroid glands—they will usually “find themselves” as the thyroid is elevated further and further onto the anterior surface of the trachea (Fig. 4).
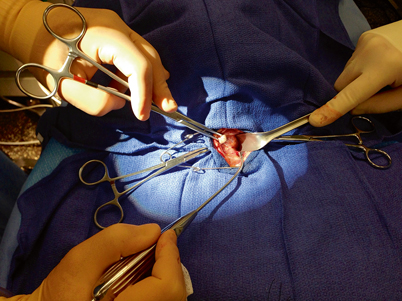
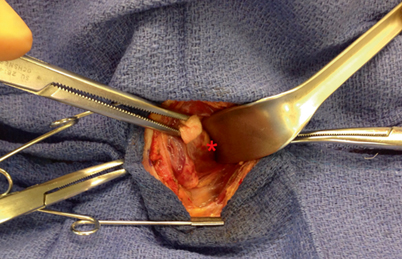

Fig. 3
As the thyroid gland is being elevated and rolled over the trachea, and the strap muscles are retracted laterally, a pediatric suction cannula is used to bluntly and atraumatically dissect the filmy attachments off of the lateral and posterior aspect of the thyroid capsule

Fig. 4
A parathyroid adenoma ( red asterisk) is visualized deep to the posterior aspect of the thyroid capsule
Parathyroid Localization
The sequence of intraoperative maneuvers used to identify the parathyroid gland depends on whether the upper or lower gland is the target . Upper glands, which on sestamibi scan often appear medial to the upper pole of the thyroid (Fig. 5), tend to lie quite deep behind the upper and midpole regions of the thyroid. Their identification and removal often requires extensive medial mobilization of the thyroid gland, and, rarely, the blunt opening of Reeve’s space between the upper pole of the thyroid and the cricothyroid muscle. On occasion, division of superior thyroid vessels or the middle thyroid vein can facilitate sufficient mobilization of the upper pole of the thyroid to identify these glands.
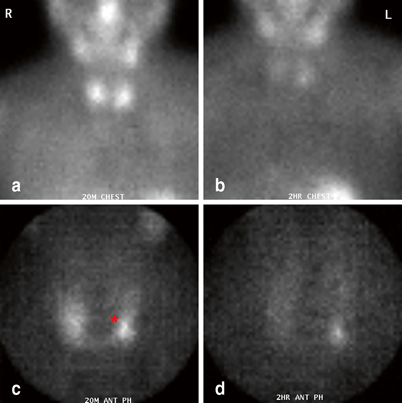

Fig. 5
Immediate (a, c) and delayed (b, d) sestamibi images (global views (a, b) and pinhole images (c, d)) demonstrating a left upper parathyroid adenoma (red asterisk). Note the relatively medial position of the parathyroid mass on image c and d
The lower glands (Fig. 6) generally require less mobilization of the thyroid gland to identify. They are sometimes visible immediately upon reflecting the sternothyroid muscle off of the thyroid gland. If no gland is identified, I search lower in the anterior mediastinum. If thymectomy is required, I divide the thyrothymic ligament, and grasp and retract the cervical portion of the thymus cephalad as the loose areolar tissues are pushed down. Occasional perforating vessels are clipped or divided using bipolar cautery. Usually, the cervical thymus will thin out and cleave itself. Otherwise, especially if sufficient thymic tissue has been mobilized to include the entire parathyroid, it may be ligated or clipped, and divided.
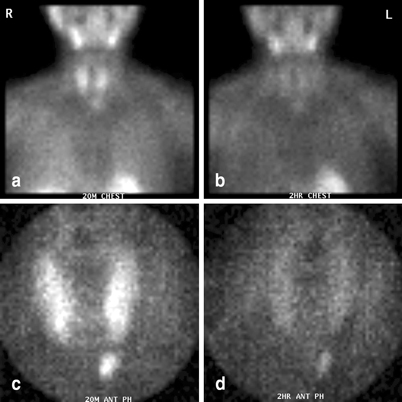
Fig. 6
Immediate (a, c) and delayed (b, d) sestamibi images (global views (a, b) and pinhole images (c, d)) demonstrating a left lower parathyroid adenoma. At surgery, the adenoma was identified within the thymus gland
In cases where a parathyroid gland remains elusive, it is often helpful to reflect on the gland that has already been found on that side and its relationship with the recurrent laryngeal nerve. If the gland that was already identified was in a location anterior to the nerve, then a more comprehensive search is undertaken for the upper gland concentrating on the region of the tracheoesophageal groove, with extension of the field to include the carotid sheath, retropharyngeal, and retroesophageal spaces extending both above and below the thyroid gland. In cases where the lower gland is missing (i.e., the identified gland was posterior to the nerve), the search focuses on the thyrothymic ligament and thymus, and the accessible portion of the superior mediastinum are also explored. In all cases of a missing parathyroid, the carotid sheath should be opened and explored from the root of the neck to the skull base. If thyroid nodules are present on ultrasound, thyroid lobectomy should be done. Proximal ligation of the inferior thyroid artery has not been demonstrated to be curative, but may devascularize the missing parathyroid gland. Median sternotomy is not indicated unless the operation is being performed for life-threatening hypercalcemia. In the majority of revision parathyroidectomies, the “missing parathyroid” will eventually be found in a normal anatomic position. Therefore, following this progression, even if the gland has not been found, the operation should be concluded, and the patient should be reimaged and planned for revisional surgery [17] .
Lateral Approach
The lateral approach to the parathyroids can be taken either through a Kocher-type skin incision or through a lateral incision centered on the anterior border of the sternocleidomastoid (SCM) muscle. Regardless of the skin incision, the subplatysmal plane is developed, and following this, the anterior border of the SCM is dissected. The plane between the anterior border of the SCM and the strap muscle and thyroid complex is then entered, retracting the SCM and carotid artery laterally and the strap muscles and thyroid gland medially. It is imperative to keep the dissection medial to the carotid artery, which may slip medially from under the retractor and confuse the dissection field. This approach, which offers direct access to the posterior tracheoesophageal groove, is most useful in redo cases involving an upper gland.
The main advantage of this approach is also its greatest weakness: The direct access to the tracheoesophageal groove means that the parathyroid can be identified more directly, but it also means that the recurrent laryngeal nerve is encountered more directly than when the central approach is used. Because this approach leads to the deep aspect of the tracheoesophageal groove, it takes extra effort to expose and remove a lower parathyroid, which is located in a more anterior plane. Additionally, this approach is useful for a planned unilateral exploration.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree